Nanoparticle-Based Drug and Gene Delivery for Tumor Targeting
Takeshi Mori, Akihiro Kishimura, Yoshiki Katayama*
Department of Chemistry and Biochemistry, Faculty of Engineering, Kyushu University, Japan
Material Matters™, 2019, 14.3
- Augmentation of the EPR Effect
- Stimuli-Responsive Systems for Nanoparticle-Based Delivery
- Conclusions
Cancer is a leading cause of death worldwide. If detected early, surgical resection can be a highly effective treatment. But for metastatic cancers or tumors in difficult-to-resect sites, chemotherapy remains the most promising option. However, the response rate of tumors to chemotherapy can be as low as 10–20%, and limited by low delivery efficiency1 and there are severe side effects caused by drug delivery to other sites, even for targeted therapies. In this sense, better tumor-targeting drug or gene delivery strategies are needed to deliver significant improvements for patients.
In 1986, Maeda et al. discovered tumor neovasulature shows enhanced leakage, or extravasation of serum albumin or other molecules.2 Driven by incomplete endothelium formation and poor development of lymphatics, macromolecules of more than 40 kDa leak from the blood vessel and selectively accumulate in tumor sites. This phenomenon, known as enhanced permeability and retention (EPR) effect, allows for a number of versatile strategies to create tumor-selective drug delivery systems,3 and nanoparticles have been investigated as tumor-targeting drug delivery systems utilizing this effect. Nanosized anticancer drugs are large enough to escape renal clearance and avoid penetration of the tight endothelial junctions of normal blood vessels, yet small enough to extravasate in leaky tumor vasculatures and selectively accumulate in tumor tissues, making them ideal for EPR-based selective anticancer therapy.4 This paper discusses recent advances in these systems and strategies.
Augmentation of the EPR effect
The EPR effect is one of the most effective ways to target a tumor site, and effective accumulation of nanoparticles at tumor sites is commonly observed in murine model systems. However, only 1% of human clinical studies have demonstrated effective use of the EPR effect by nanoparticulate delivery systems.5 In fact, the poor progression of the lymph system in many tumors causes high interstitial fluid pressure which suppresses extravasation. As a result, selective delivery of a drug to the tumor site by the EPR effect using nanoparticle systems is hindered. To resolve this issue, other strategies have been developed to augment EPR effect, thereby allowing selective accumulation of therapeutic agents at tumor sites.
As previously discussed, an effective delivery system approach to enable extravasation is to overcome the high interstitial fluid pressure at the tumor site. In normal tissues this pressure is usually 3–10 mm Hg, but can be as high as 40–60 mm Hg in tumor tissue.6 Salnikov et al. used Prostaglandin E1 for fluid pressure control, and reported a 40% enhancement of 5-FU accumulation at tumor sites (Figure 1A).7 Another strategy is to increase systolic blood pressure, and Maeda et al. reported angiotensin-induced hypertension chemotherapy, which led to increased nanoparticle accumulation in tumors (Figure 1B).8 However, selectively controlling blood flow in tumor vasculature is challenging, and such methods can induce systemic adverse effects such as a cardiovascular event.9 Nanoparticles that offer controlled release of vasodilators (medications that dilate blood vessels) are a promising resolution. For example, spontaneous nitric oxide (NO) releaser (NOC-18) encapsulated liposomes have been investigated for enhancing the EPR effect.10 After liposomes are absorbed by tumor sites by the EPR effect, NOC-18 gradually releases NO which, in turn, induces vasodilation, thereby achieving selective vasodilation in cancer neovasculatures. This resulted in enhanced accumulation of drug accumulation selectively at tumor sites (Figure 1C). Similarly, researchers used S-nitorosothiol-incorporated serum albumin as the macromolecular vasodilator11 to achieve localized vasodilation and two to five fold enhanced particle accumulation at tumor sites.

Figure 1.Strategies of Enhancement in EPR effect. A) PEG1 administration causes amplification of blood vessel permeability. B) Angiotensin administration cause systemic high blood pressure, but cancer neovasculature has poor response to angiotensin. C) NO-releasing carriers causes cancer neovasculature-specific vasodilation.
Stimuli-responsive systems for nanoparticle-based delivery
Although EPR-enhanced systems improve the pharmacological distribution of a therapeutic drug or gene while also decreasing the effective dosage required, sometimes there can also be higher accumulation in the liver or other organs. To address this issue, stimuli responsive drug release systems that occur selectively in the target tumor tissue are being investigated. Such systems aim to increase the drug concentration ratio between the target site and other normal organs and tissues.
Several different stimuli-responsive carriers have been developed for improved drug release specifically in tumor sites. Since solid tumors form a characteristic microenvironment that is quite different from normal tissue, many of these systems use tumor microenvironment factors as a trigger to release the drug payload. For example, tumor tissue initially produces high concentrations of lactic acid, lowering the pH of the tumor microenvironment to 5.85–7.68, from a normal mean pH value of 7.52.12 Vigorous proliferation of tumor cells tends to cause hypoxia, which leads to the expression of a special transcription factor (HIF-1) to counteract it. Tumor cells also contain a much higher level of glutathione (GSH, 2–10 mM) than normal cells, in order to vanish reactive oxygen species produced by their high metabolic activity.13 The tumor microenvironment also induces various enzymes including matrix metalloproteases (MMP), β-glucuronidase, hyaluronidase and urokinase plasminogen activator, as well as abnormal activation of some kinases and transcription factors.1 These proteins are essential to for tumor progression, metastasis, and invasion. Any one of these factors could be used to stimulate of drug release in a tumor selective system. Some examples of such stimuli-responsive systems are described below.
pH-responsive system
The lower pH at tumor sites is the most commonly exploited stimuli in tumor-specific drug delivery systems (DDS). In addition to the acidic pH found in tumor tissues, endosomal pH (pH 5.5–6) can also be used to accelerate drug release from carriers. Because nanoparticles are taken up by tumor cells by endocytosis, rapidly decreased endosomal pH can also be used to trigger drug release in the cytosol, causing a rapid increase of drug concentration in the cytosol. Thus, pH-responsive carriers cause a rapid increase of drug concentration near and inside tumor cells.
There are two major strategies used to design pH-responsive carriers. One is the use of pH-responsive cleavage linkers in carrier molecules. Imine, cyclic orthoester, and acetal bonds are all hydrolysable at mild acidic pH (Figure 2A).15 These cleavable linkers can be incorporated into polymeric nanocarriers, allowing them to degrade and release therapeutic agents in the lower pH environment of the tumor. For example, a triblock copolymer consisting of PEG-oxime-tethered polycaprolactone-PEG triblock copolymer forms a core-shell type polymer micelle16 in aqueous solution. The hydrophobic core of the nanoparticles rapidly decomposed at low pH in tumor tissue and triggered the release of encapsulated drugs. Another strategy is to use a weak base that can be protonated at lower pH. Chen et al. coated doxorubicin (DOX)-containing mesoporous silica nanoparticles with folic acid-PEG modified polydopamine.17 Protonation of the coating polymer at acidic pH destabilized the coating layer and induced the release of DOX. Another example is methoxy-PEG-b-poly ε-caprolactone-b-poly glutamate triblock copolymers, which form vesicles (polymersomes) in aqueous solution. Structural disintegration occurs at endosomal pH with the protonation of poly glutamate chain.18 As for gene delivery, the pH buffering effect is commonly adopted for the acceleration of endosomal release of gene cargo (Figure 2B). Protonation of the gene delivery carriers inside the endosomes induced the influx of counterions and water, leading to osmotic pressure increase and eventually endosome breakage and content release. This is known as the proton sponge effect.19 Polyethyleneimine (PEI) derivatives are the most common example of such gene carriers.20
pH-dependent changes in peptide conformation can also be used to create pH-responsive systems. Bacteriorhodopsin C helixderived peptides assume cell-penetrating a-helix conformation at weakly acidic conditions, enhancing cell uptake of the nanoparticles in tumor tissue.21

Figure 2.pH-responsive carriers. A) Acid-sensitive linkage inserted polymer micelle. B) Proton sponge effect of drug carriers using weak base units.
Redox-responsive systems
High glutathione (GSH) concentration at tumor sites is another common signal for redox-responsive DDS (Figure 3B). Polymeric carriers containing disulfide linkages have been developed for drug and gene delivery. For example, PEG-b-polyl-lysine-SS-polycaprolactone was designed and used to form polymeric vesicles and encapsulate DOX and Verapamil into its inner aqueous phase and hydrophobic shell, respectively.23 The disulfide bonds inside vesicles could be reversibly cleaved in a reductive environment, thus triggering the release of the encapsulated drugs. Similarly, researchers have shown that polymersome formed from poly-Z-L-lysine-SS-PEG-SS-poly-Zl-lysine triblock copolymer selectively released DOX in tumor cells, whereas the release was suppressed in normal cells.24 This GSH-based redox-responsive strategy has also been adopted for gene delivery systems. Researchers have demonstrated that nanoparticles consisting of PEI-b-cyclodextrin, adamantyl-SS-PEG, and adamantyl-PEG-SP94 (anti-CD7 antibody) could preferentially deliver miR34 (microRNA) to LM3HCC cancer cells and suppress their proliferation.25
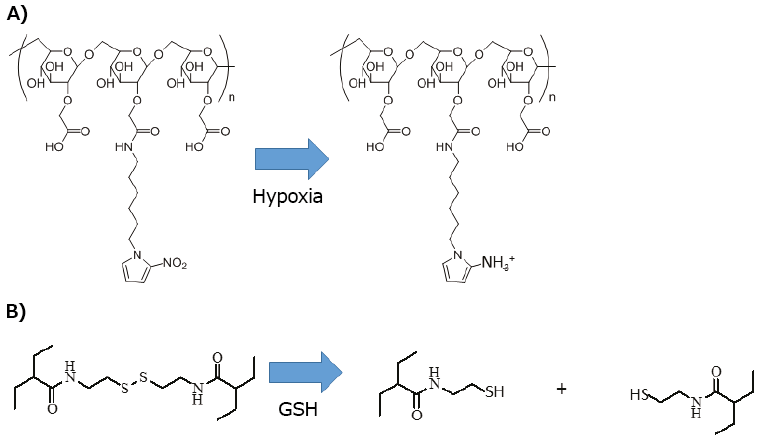
Figure 3.Redox-responsive carriers. A) Nitro-imidazol is reduced to amino-imidazol in hypoxic condition in tumor tissue. B) Didulfide linkage is cleaved with reduction by glutathione.
Enzyme-responsive systems
Many tumor tissues have high MMP-2/9 activity,26 which has also been used as a signal for designing enzyme-responsive DDS. Researchers have developed solid lipid nanoparticles consisting of glycerol-monostearate, capric triglyceride, and phosphatidylcholone with a gelatin coating layer for the delivery of drugs.27 At the tumor site, high MMP level degraded gelatin efficiently and induced the release of encapsulated drugs (Figure 4A), delivering them preferentially to the tumor site over normal tissue. Also, MMP cleavable linkers were used in core-shell type polymer micelles (Figure 4B). Specifically, hydrophilic shells such as PEG or avidin were connected with the core through an MMP substrate peptide linker, which was susceptible to high MMP concentration at the tumor site. At the tumor sites, the cleavage of the hydrophilic shell induced by high MMP concentration disrupted the drug nanocarrier, resulting in localized drug release at tumor tissues.28,29 In another case, PEG2000-MMP2 substrate-PEI1800-dioleylphosphatidylethanolamine was capable of co-encapsulating both Paclitaxel and siRNA. The cleavage of PEG by MMP2 was shown to accelerate the siRNA release at cancer sites (Figure 4C).28 Also, PEG-MMP substrate cell penetrating peptide (CPP)- phospholipid was inserted onto a liposome membrane forcimproved cellular uptake.30 The PEG part was cleavable by MMP in tumor tissue and the newly-exposed CPPs contributed to cell uptake of the particles (Figure 4D). Using the same concept, another group designed cationic CPP connecting oligoanionic peptides using an MMP substrate as the delivery vehicle. The electrostatic interaction between the CPP and the anionic sequence shielded the cell penetrating activity. Removal of the anionic sequence in the tumor by MMP cleavage also improved cellular uptake (Figure 4E).31
Intracellular enzymatic activity is also useful for cancer cell-specific payload release. Various protein kinases play essential roles in tumor growth, survival and angiogenesis,32 and are by definition absent in normal cells, so can be used as drug-releasing stimuli. For example, a cancer targeting gene delivery system has been designed using a protein kinase signal (Figure 4F).33 Specifically, protein kinase-specific cationic peptide substrates were chosen and used to construct grafted polymers. The cationic peptide grafted polymer formed nanosized polyplexes with genetic materials through electrostatic interactions. At tumor sites, polymer side chains are phosphorylated by the abnormally-activated protein kinase. The negative charges introduced by phosphate groups weakened the interaction between the carrier and DNA, thereby triggering the release of the gene cargo. Furthermore, the kinase specificity of the delivery vehicle can be easily tuned by modifying the pendant peptide sequence. Various gene delivery systems responding to different protein kinases, such as protein kinase A, Src, I-k-kinase, Akt or protein kinase Ca (PKCa) have been developed for different tumor site targeting applications.34,35

Figure 4.Enzyme-responsive carriers. A) Gelatin-coated solid lipid nanoparticle. B) PEG removal with MMP in polymer micelle. C) PEG removal and CCP expose with MMP in lipid coated nanoparticle. D) PEG removal with MMP in liposome. E) PEG removal and CCP expose with MMP in liposome. F) Protein kinase-responsive gene release.
Physical stimuli-responsive system
Physical stimulations can also be used for stimuli-responsive DDS. Yang et al. reported on photo-responsive liposomes. They developed drug carrier molecules using a CPP peptide and phosphatidylethanolamine, with photo-cleavable linkers. CPP is shielded in blood circulation and normal tissue by its anionic sequence. Localized drug release was achieved by photo-irradiation of the tumor site, which cleaved the 2-nitro-4,5-dimethoxybenzyl group, inducing another intramolecular reaction, causing removal of the anionic peptide moiety and release of the therapeutic agent (Figure 5).36 Despite the fact that light is a spatially and temporally controllable stimuli, this cleavage reaction requires near UV (usually less than 360 nm) light which cannot penetrate tissue.37 For penetration into deeper tissue, a photocleavable reaction in near IR (650–900 nm) region is needed.38

Figure 5.Photo-responsive carrier. Photo-irradiation causes the cleaving of oligo-anion peptide and CCP expose in liposome.
Multi stimuli-responsive system
More sophisticated multi stimuli-responsive DDS are also being developed to achieve precise tumor targeting. An et al. proposed pH and redox dual-responsive drug delivery systems (Figure 6A).39 They designed a novel poly(ethylene glycol)-block-poly(disulfide histamine) copolymer as a nanomaterial drug delivery carriers. When the nanocarriers reach the tumor tissue via the EPR effect, the imidazole moieties are protonated at the weakly acidic pH, improving the endocytotic cellular uptake efficiency. The high concentration of GSH reduces the disulfide bonds in the polymer and disrupts the nanoparticle structure, resulting in rapid increase of the drug concentration near the nucleus of tumor cells.

Figure 6.Multistimuli-responsive carriers. A) pH and redox responsive carrier. B) pH and protein kinase responsive carrier.
In addition, pH and kinase dual-responsive systems have been designed by simply converting the polymer backbone from a neutral polymer to PEI (Figure 6B).40 The additional proton sponge effect results in an improved endosome escape efficiency of the polyplex with DNA. Consequently, PKCa delivery increases from 10 to 500 times when compared to the expression level in a non-PKCa responsive system using a control peptide.
Conclusion
Drug and gene delivery systems using nanoparticles have great potential to target the delivery of therapeutic or diagnostic agents. However, there are still issues in clinical applications because of poor methodology, instability, biocompatibility, degradability, complicated formulations, and lack of standardization. Also, nanoparticle systems sometimes show unexpected toxicity or inefficiency. In fact, some systems have failed clinical trials due to unexpected instability or lack of tumorspecific targeting despite their superiority to ordinary therapeutics in murine models. Developing new materials for drug carriers, as well as new evaluation methods and strategies, are crucial steps for the continued advancement of human tumor targeting.
References
Para continuar lendo, faça login ou crie uma conta.
Ainda não tem uma conta?