Staining for Fungi
Vinnie Della Speranza, MS, HTL(ASCP)1, Rena Fail, HT(ASCP)1
1 Medical University of South Carolina, Charleston, SC
This article has been reprinted with permission from HistoLogic, vol 38 no. 1, 2005.
Importance of Fungal Stains
Fungal stains remain an important tool in the histology laboratory’s diagnostic arsenal for identifying infectious microorganisms. Dermatomycoses, or superficial fungal infections involving the skin, are rarely a serious health problem in man; however, systemic mycoses with such organisms as Cryptococcus neoformans and Candida albicans can cause life-threatening infections, especially among those who are immune suppressed. Therefore, fungal stains are often ordered with some urgency by the surgical pathologist when hints of fungal infection are observed with the H&E stain.
Schiff's Reagent and Hexamine Silver Solutions
Most fungi are fairly large and their walls are rich in polysaccharides (1,2-glycol groups), which can be demonstrated with Schiff's reagent or hexamine (methenamine) silver solutions.1 Both techniques rely on the use of an oxidizer (periodic acid or chromic acid) to create aldehyde binding sites for the Schiff or silver ions.The Grocott's silver (GMS) stain is probably the most widely used fungal stain, however, the somewhat capricious nature of silver staining may challenge even the most experienced of histology practitioners. Insufficient silver impregnation, overstaining that results in high background staining, or undesirable precipitate may all contribute to difficulties in finding stained fungal organisms. Proper determination of staining endpoint with a microscope, in many instances, is essential to a successful outcome.
As a result, some pathologists will routinely order a periodic acid-Schiff (PAS) stain in the hope of attaining a rapid and more foolproof stain. This method is among the most frequently employed techniques in histology laboratories. It is relatively simple to perform and reliable, even in inexperienced hands. The PAS stain will reliably demonstrate the polysaccharide-laden wall of most fungal organisms, with the exception of Histoplasma capsulatum.2
Unintended Dying of Polysaccharide Components
One drawback to employing the PAS for fungal staining, however, is that it will stain polysaccharides wherever they appear in tissues. This method will stain many intracellular components in various types of normal cells, from a wide variety of animal species. Some of these intracellular components are listed below3:
- glycogen
- neutral mucosubstances
- some epithethial sulfomucins and sialomucins
- colloid of the thyroid
- basement membranes
- reticular fibers
- paneth cell granules
- adrenal chromaffin cells
- neuronal glycolipids
- intranuclear inclusions in vas deferens
- afibrillar cells of arterioles of kidney
- granules within reticuloendothelial cells
- salivary glands
- gastric and Brunner's glands
- mucin from respiratory tract
- acinar and endocrine glands, including the prostate in man
Staining of these elements in some tissues may be distracting when trying to find PAS-stained fungal organisms. As a result, pathologists who utilize the PAS for fungal staining will often request the stain with diastase digestion in an attempt to minimize staining structures of little or no interest.
Oxidizing Agents in Stains
Interestingly, few histology practitioners question the use of the oxidizer periodic acid in the PAS stain, yet we use the oxidizer chromic acid in the GMS stain for fungi. In fact, if we were to substitute periodic acid for chromic acid in the GMS stain, essentially we will have performed the Jones’ method,which specifically stains basement membranes and reticular fibers.
Yes, the Jones’ technique can demonstrate fungi; however, it will also stain many other structures, making the discovery of fungal organisms much more difficult. Whether we are using Schiff or silver stain, the weaker periodic acid oxidizer creates aldehyde binding sites in many more tissue structures. In this example, we can see how the selection of an oxidizing agent can significantly determine which structures will be stained. This choice will maximize the probability of achieving the desired results.
These observations were actually quite well known a generation or two ago. However, this older, yet still important information can sometimes be overlooked. Modern histology practitioners can use this information today to guide surgical pathologists in selecting the most appropriate staining method available.
Bauer (1933) is credited with the earliest report that the oxidation of sugars (1,2-glycols) with chromic acid creates aldehydes that will bind the Schiff molecule.4 Many others, including Hotchkiss, McManus (1960), Spicer, Pearse, and Lillie continued to study the aldehyde-Schiff reaction using various oxidizers. It became well understood that the strength of the oxidizer, the duration of exposure, and the density of sugars in the tissue structures of interest determine the location where the Schiff molecule will bind.
Both chromic acid and potassium permanganate, which are stronger oxidizers than periodic acid, also produce aldehydes in polysaccharides, but they further attack and destroy those aldehydes if given sufficient time. "Because of the further oxidation of aldehyde by chromic acid,less density of aldehyde reaction is achieved and structures with fewer glycol groups are not demonstrated, such as basement membranes and collagenous and reticular fibers."5 This statement by Lillie tells us why chromic acid is the better oxidizer when staining fungi, since these organisms have a greater density of sugar (polysaccharides) in their cell wall.
While chromic acid continues further oxidation of all polysaccharides, complete conversion in the areas of heaviest concentration takes longer, allowing these to remain reactive with Schiff (or silver).2 Since the walls of fungi are particularly rich in 1,2-glycol groups, they stain more intensely than most other reactive components in the tissue section. The contrast between fungi and background is often more distinct than what is seen in duplicate sections stained by the PAS reaction3 (Figures 1 and 2). This discussion suggests that if fungi are present in your tissue section, they are less likely to be missed when a stronger oxidizer like chromic acid is used.
Recommendation: Chromic Acid followed by Schiff
Work in our laboratory has convinced us that using chromic acid as oxidizer, followed by Schiff, yields results superior to PAS for fungal staining, as background staining is greatly decreased. This approach is as simple as doing a typical PAS, yet avoids the need to perform a diastase digestion, which adds unnecessary work and expense to fungal staining. The method reported by Casella3 utilizing potassium permanganate as oxidizer is another suitable alternative to periodic acid and yields similar results to chromic acid when staining fungi.
Interestingly, chromic acid-Schiff (CAS) is remarkably similar to the method by Gridley6 that can be found in many histology texts except that Gridley’s technique also requires the use of aldehyde fuchsin following Schiff. This is done to achieve more intense staining.7 The aldehyde fuchsin acts as an aldehyde and occupies uninvolved linkages of the Schiff reagent, thus reinforcing the depth of the stain.2 While we have confirmed that aldehyde fuchsin does in fact intensify the stained organisms, we speculate that the Gridley method is not widely used today because the aldehyde fuchsin stain requires the use of paraldehyde, a controlled substance that may be problematic to obtain in some laboratories.
In conclusion, the next time your pathologist requests a PAS for fungus, we recommend that you suggest the CAS as a much better alternative. It makes little sense to utilize an oxidizer that will create aldehydes in undesirable locations only to have to use an enzyme to try to eliminate them later. Better to avoid creating them in the first place. A simple demonstration with parallel stained sections, one stained with PAS and the other with CAS, will convince even the most skeptical observer.
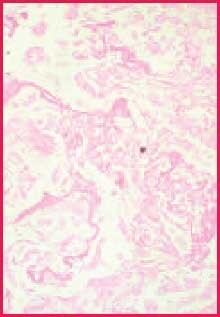
Figure 1. Positive fungus control. PAS stain, 400X
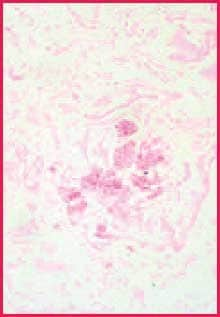
Figure 2. Positive fungus control. Chromic acid-Schiff stain, 400X
References
Para continuar lendo, faça login ou crie uma conta.
Ainda não tem uma conta?