Selective Metalations using i-PrMgCl·LiCl and s-BuMgCl·LiCl
Aaron Thornton
While halogen-metal exchange reactions are among the most common methods for preparing reactive organometallic reagents, Li-halogen exchange reactions typically require low temperatures and offer limited compatibility with other functionalities. On the other hand, Mg-halogen exchange reactions require higher temperatures and are often prone to elimination side reactions. To address these issues, Knochel and coworkers have found that the use of salt additives increase both the rate and the efficiency of this Mg-halogen exchange reaction. The most effective reagents are generated with R-MgCl (R = i-Pr, s-Butyl) and 1.0 equiv of LiCl. The increased reactivity of these aptly named TurboGrignards may be due to the breakup of polymeric aggregates known to exist in classical solutions of Grignard reagents. TurboGrignards allow for the conversion of a variety of functionalized and highly sensitive substrates, including those containing CO2R, CN, OMe, and halogen moieties, to their corresponding functionalized organometallic derivatives. While rate enhancements are observed with TurboGrignards, this increased reactivity does not have a negative impact on the overall scope of the reaction, permitting transformations to occur in the presence of a broad range of functional groups (Table 1).1
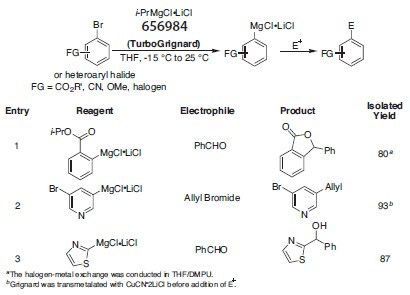
Table 1:Aryl/heteroaryl Grignard reagents prepared using i-PrMgCl·LiCl and reactions with electrophiles (656984).
Advantages of TurboGrignards:
- Increased functional group compatibility
- Mild reaction conditions
- Minimal side reactions
- Allows for the large-scale production of reactive Grignard reagents
References
Para continuar lendo, faça login ou crie uma conta.
Ainda não tem uma conta?