ELISA Protocols
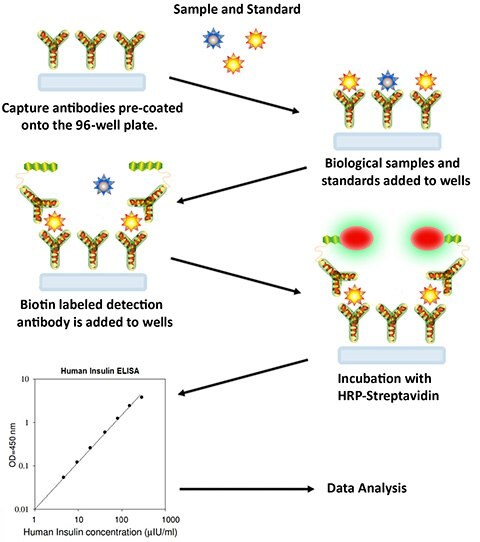
Sandwich Assay Procedure
- Bring all reagents and samples to room temperature (18 - 25 °C) before use. It is recommended that all standards and samples be run at least in duplicate.
- Add 100 µL of each standard and sample into appropriate wells. Cover wells and incubate for 2.5 hours at room temperature or overnight at 4 °C with gentle shaking.
- Discard the solution and wash 4 times with 1X Wash Solution. Wash by filling each well with Wash Buffer (300 µL) using a multi-channel Pipette or autowasher. Complete removal of liquid at each step is essential to good performance. After the last wash, remove any remaining Wash Buffer by aspirating or decanting. Invert the plate and blot it against clean paper towels.
- Add 100 µL of 1x prepared Detection Antibody to each well. Cover wells and incubate for 1 hour at room temperature with gentle shaking.
- Discard the solution. Repeat the wash procedure as in step 3.
- Add 100 µL of prepared Streptavidin solution to each well. Cover wells and incubate for 45 minutes at room temperature with gentle shaking.
- Discard the solution. Repeat the wash as in step 3.
- Add 100 µL of TMB One-Step Substrate Reagent (Item H) to each well. Cover wells and incubate for 30 minutes at room temperature in the dark with gentle shaking.
- Add 50 µL of Stop Solution (Item I) to each well. Read absorbance at 450 nm immediately.
Phosphorylation Assay Procedure
- Bring all reagents to room temperature (18 - 25 °C) before use. It is recommended that all samples or Positive Control should be run at least in duplicate.
- Add 100 µL of each sample or positive control into appropriate wells. Cover well with plate holder and incubate for 2.5 hours at room temperature or overnight at 4 °C with shaking.
- Discard the solution and wash 4 times with 1x Wash Solution. Wash by filling each well with Wash Buffer (300 µL) using a multi-channel pipette or autowasher. Complete removal of liquid at each step is essential to good performance. After the last wash, remove any remaining Wash Buffer by aspirating. Invert the plate and blot it against clean paper towels.
- Add 100 µL of prepared 1X biotinylated anti-phosphotyrosine antibody to each well. Incubate for 1 hour at room temperature with shaking.
- Discard the solution. Repeat the wash as in step 3.
- Add 100 µL of prepared 1X HRP-Streptavidin solution (see Reagent Preparation step 6) to each well. Incubate for 45 minutes at room temperature with shaking.
- Discard the solution. Repeat the wash as in step 3.
- Add 100 µL of TMB One-Step Substrate Reagent (Item H) to each well. Incubate for 30 minutes at room temperature in the dark with shaking.
- Add 50 µL of Stop Solution (Item I) to each well. Read at 450 nm immediately.

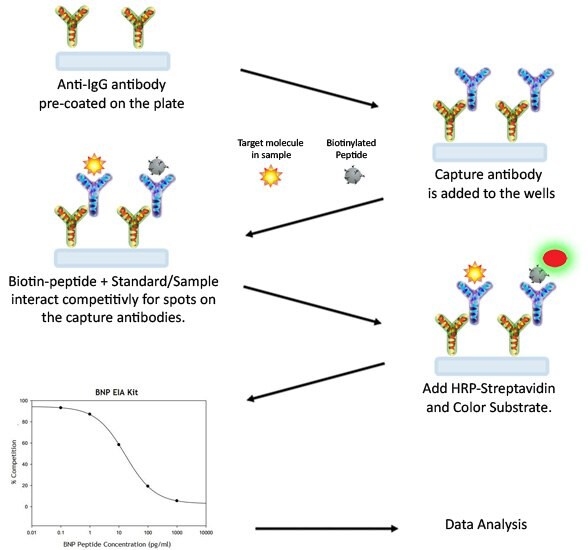
EIA Assay Procedure
- Keep kit reagents on ice during reagent preparation steps. It is recommended that all standards and samples be run at least in duplicate.
- Add 100 μL anti-“target” antibody to each well. Incubate for 1.5 hours at room temperature with gentle shaking (1-2 cycles/sec). You may also incubate overnight at 4 °C.
- Discard the solution and wash wells 4 times with 1x Wash Buffer (200-300 μl each), Washing may be done with a multichannel pipette or an automated plate washer. Complete removal of liquid at each step is essential to good assay performance. After the last wash, remove any remaining Wash Buffer by aspirating or decanting. Invert the plate and blot it against clean paper towels.
- Add 100 μL of each standard, positive control and sample into appropriate wells. Be sure to include a blank well (Assay Diluent only). Cover wells and incubate for 2.5 hours at room temperature with gentle shaking (1-2 cycles/sec) or overnight at 4 °C.
- Discard the solution and wash 4 times as directed in Step 3.
- Add 100 μL of prepared HRP-Streptavidin solution to each well. Incubate for 45 minutes with gentle shaking at room temperature. It is recommended that incubation time should not be shorter or longer than 45 minutes.
- Discard the solution and wash 4 times as directed in Step 3.
- Add 100 μL of TMB One-Step Substrate Reagent (Item H) to each well. Incubate for 30 minutes at room temperature in the dark with gentle shaking (1-2 cycles/sec).
- Add 50 μL of Stop Solution (Item I) to each well. Read absorbances at 450 nm immediately.
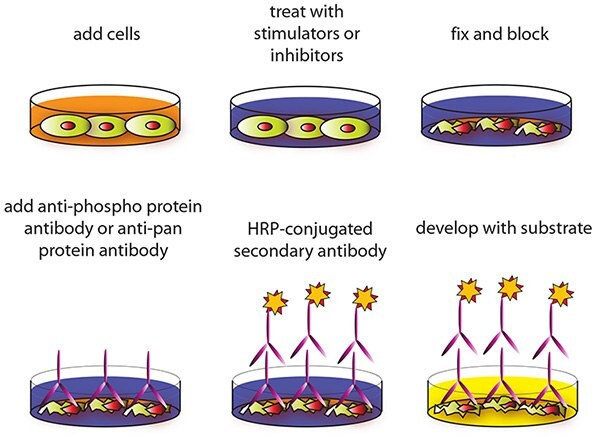
Cell-based Assay Procedure
NOTE: ALL incubations and wash steps must be performed under gentle rocking or rotation (~1‐2 cycles/sec).
- Design your experiment.
OPTIONAL: If seeding HUVECs, HMEC‐1 or other loosely attached cells, coat the Uncoated 96‐Well Microplate (ITEM A) by adding 100 μL poly‐L‐Lysine (recommended Sigma-Aldrich Product No. P4832) into each well and then follow manufacturer’s instructions. A pre‐coated CellBIND® microplate or other poly‐lysine treated tissue culture plate may be used in place of ITEM A. - Seed 100 μL of 10,000 to 30,000 cells into each well of the Uncoated 96‐ Well Microplate (ITEM A) provided and incubate overnight at 37 °C with 5% CO2.
NOTE: The optimal cell number used will vary on the cell line and the relative amount of protein phosphorylation. More or less cells may be used but this must be determined empirically. NOTE: The cells can be starved ~4‐24 hours (depending on cell line) prior to treatment with inhibitors or activators. - Apply various treatments, inhibitors (such as siRNA or chemicals) or activators according to manufacturer’s instructions and incubate for the desired time points.
NOTE: It is recommended to dissolve inhibitors or activators into serum‐free cell culture medium before treating the cells (unless otherwise stated in the manufacturer’s instructions.) - Discard the cell culture medium by flipping the microplate upside down and gently tapping the bottom of the microplate over a sink.
- Wash by pipetting 200 μL of the prepared 1X Wash Buffer A (ITEM B) into each well. Discard the wash buffer (same as step 4) and wash 2 more times for a total of 3 washes using fresh wash buffer each time. After the final wash, gently blot the microplate onto a paper towel to remove any excess/remaining buffer.
NOTE: To avoid cell loss, do not pipette directly onto the cells. Instead, gently dispense the liquid down the wall of cell culture wells. Also avoid the use of vacuum suction or too forcefully tapping the microplate when discarding any solution. - Add 100 μL of Fixing Solution (ITEM D) into each well and incubate for 20 minutes at room temperature.
NOTE: The fixing solution is used to permeabilize the cells. - Repeat wash step 5.
- Add 200 μL of prepared 1X Quenching Buffer (ITEM E) into each well and incubate 20 minutes at room temperature.
NOTE: The quenching buffer is used to minimize the background response. - Wash 4 times with 1X Wash Buffer A.
- Add 200 μL of the prepared 1X Blocking Buffer (ITEM F) into each well and incubate for 1 hour at 37 °C.
- Wash 3 times with the prepared 1X Wash Buffer B (ITEM C).
NOTE: If needed, the microplate may be stored at ‐80 °C for several days after this wash. - Add 50 μL of the prepared 1X primary antibody (ITEM G‐1, G‐2, G‐3, H‐ 1, H‐2 or H‐3) into each corresponding well and incubate for 2 hours at room temperature.
- Wash 4 times with 1X Wash Buffer B.
- Add 50 μL of the prepared 1X HRP Conjugated secondary antibody (ITEM I‐2) into each well and incubate for 1 hour at room temperature.
- Repeat step 13.
- Add 100 μL of the TMB Substrate (ITEM J) into each well and incubate for 30 minutes at room temperature in the dark.
- Add 50 μL of the Stop Solution (ITEM K) into each well. Read at 450 nm immediately.
These protocols are intended for use as a guideline only. Please reference the detailed technical bulletin for each kit for specific instructions.
Faça login para continuar
Para continuar lendo, faça login ou crie uma conta.
Ainda não tem uma conta?