CRM03054
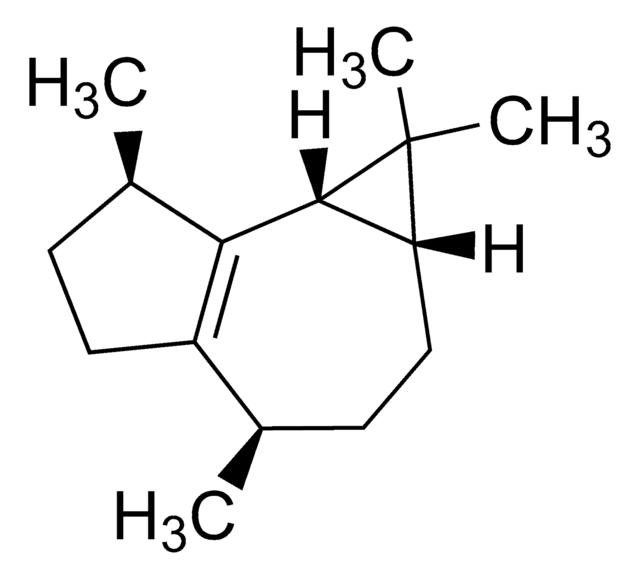
Tetramethrin
mixture of stereoisomers, certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
About This Item
Recommended Products
grade
certified reference material
TraceCERT®
Quality Level
product line
TraceCERT®
shelf life
limited shelf life, expiry date on the label
composition
cis-Tetramethrin, <25.0% (minor component)
manufacturer/tradename
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
storage temp.
−20°C
SMILES string
C\C(C)=C\C1C(C(=O)OCN2C(=O)C3=C(CCCC3)C2=O)C1(C)C
InChI
1S/C19H25NO4/c1-11(2)9-14-15(19(14,3)4)18(23)24-10-20-16(21)12-7-5-6-8-13(12)17(20)22/h9,14-15H,5-8,10H2,1-4H3
InChI key
CXBMCYHAMVGWJQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Tetramethrin, a type I pyrethroid, is used worldwide to protect crops and mammals from a variety of pests such as flies, fleas, mosquitoes, headlice, and mites. Tetramethrin is a synthetic pyrethroid insecticide, that binds to voltage-gated sodium channels of insect nerves and prevents its transition from an activated (ion-conducting) to an inactivated (non-conducting) state. This leads to a permanent depolarization of axonal membranes, causing paralysis and death of an insect.
Tetramethrin is not approved for use in the EU.
The maximum residue limits (MRLs) of pyrethroids compounds in fruits and fruit juices have been set at 0.02 to 0.5 mg/kg in China (GB2763-2019), and 0.02 to 0.3 mg/kg in European Union (EU) (Part A of Annex to Reg. 396/2005), respectively.
Application
- Study of Estrogenic activity of tetramethrin in uterine CaBP-9k gene expression and a uterotrophic assay, and androgenic activity in Hershberger assay
- To synthesize bis-dihydroxyboron fluorescein complexes for developing a ratiometric fluorescence probe used in the direct detection of pyrethroid residues in fruit juices
- Separation of the four isomers of tetramethrin with ecotoxicity test and quantitative analysis in agrochemical formulations using chiral electrokinetic chromatography methodology
- Residual detection of acetanilide, pentylenetetrazole, phenacetin, and tetramethrin in porcine muscle, pasteurized milk, and eggs by liquid chromatography-electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS) method
- To test glucocorticoid-like activity of propylparaben, butylparaben, diethylhexyl phthalate, and tetramethrin mixtures in different combinations at real human exposure levels
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 2 - STOT SE 2 Inhalation
Target Organs
Nervous system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service