N6514
Neuraminidase from Vibrio cholerae
Type II, buffered aqueous solution, 8-24 units/mg protein (Lowry, using NAN-lactose)
Synonym(s):
Acyl-neuraminyl Hydrolase, Receptor-destroying enzyme, Sialidase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
Vibrio cholerae
Quality Level
type
Type II
form
buffered aqueous solution
specific activity
8-24 units/mg protein (Lowry, using NAN-lactose)
foreign activity
Protease and NAN-aldolase, present
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
General description
Calorimetry studies show that the two lectin-like domains flanking the central catalytic domain of Neuraminidase serve as the recognition and binding sites for sialic acid-containing substrates.
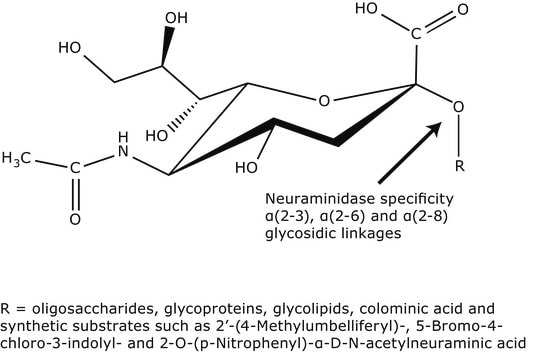
Neuraminidase enzymes are hydrolase enzymes that promote influenza virus release from infected cells and facilitate virus spread.
Application
Neuraminidase from Vibrio cholera has been used in a study to describe a five-step purification method. It has also been used in a study to investigate modification of leukemia L1210 tumor cells.
Neurminidase is used as a cell-surface probe for glycoconjugate distribution and in substrate specificity studies.
Quality
Preservative free.
Unit Definition
One unit will liberate 1.0 μmole of N-acetylneuraminic acid per min at pH 5.0 at 37 °C using NAN-lactose or bovine submaxillary mucin, unless otherwise specified. Prices based on units using NAN-lactose as substrate.
Physical form
Solution in 50 mM sodium acetate, pH 5.5, containing 0.15 M sodium chloride and 4 mM calcium chloride, 0.2 μm filtered..
Preparation Note
A further purification by affinity chromatography of our Type III.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Crennell et al.
Structure (London, England : 1993), 2(6), 535-544 (1994-06-15)
Vibrio cholerae neuraminidase is part of a mucinase complex which may function in pathogenesis by degrading the mucin layer of the gastrointestinal tract. The neuraminidase, which has been the target of extensive inhibitor studies, plays a subtle role in the
Purification and Properties of Neuraminidase from Vibrio cholera
Ada, G., et al.
Microbiology, 24, 409-421 (1961)
Increase of leukemia L1210 immunogenicity by Vibrio cholerae neuraminidase treatment.
J G Bekesi et al.
Cancer research, 31(12), 2130-2132 (1971-12-01)
Thomas P Peacock et al.
Emerging microbes & infections, 7(1), 176-176 (2018-11-08)
Avian influenza A(H9N2) viruses are an increasing threat to global poultry production and, through zoonotic infection, to human health where they are considered viruses with pandemic potential. Vaccination of poultry is a key element of disease control in endemic countries
Paul Clark et al.
Viruses, 12(10) (2020-10-24)
Polyomaviruses are small, non-enveloped DNA tumor viruses that cause serious disease in immunosuppressed people, including progressive multifocal leukoencephalopathy (PML) in patients infected with JC polyomavirus, but the molecular events mediating polyomavirus entry are poorly understood. Through genetic knockdown approaches, we
Protocols
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service