L5420
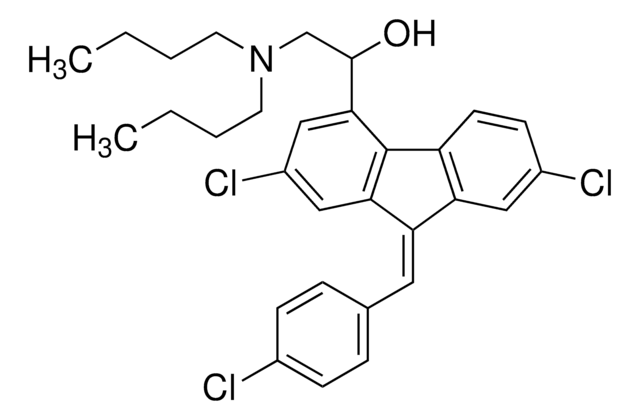
Lumefantrine
Synonym(s):
(9Z)-2,7-Dichloro-9-[(4-chlorophenyl)methylene]-α-[(dibutylamino)methyl]-9H-fluorene-4-methanol, Benflumetol, CPG-56695
About This Item
Recommended Products
Assay
≥98% (HPLC)
Quality Level
form
powder
color
yellow
solubility
DMSO: 2 mg/mL, clear (warmed)
originator
Novartis
storage temp.
room temp
SMILES string
CCCCN(CCCC)CC(O)c1cc(Cl)cc2C(=C/c3ccc(Cl)cc3)\c4cc(Cl)ccc4-c12
InChI
1S/C30H32Cl3NO/c1-3-5-13-34(14-6-4-2)19-29(35)28-18-23(33)17-27-25(15-20-7-9-21(31)10-8-20)26-16-22(32)11-12-24(26)30(27)28/h7-12,15-18,29,35H,3-6,13-14,19H2,1-2H3/b25-15-
InChI key
DYLGFOYVTXJFJP-MYYYXRDXSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
to study its effect on ex-vivo Plasmodium falciparum sensitivity using the tritiated hypoxanthine-based assay
as a standard in the quantification of combined tablet formulation using HPTLC
as a drug molecule in in vitro growth inhibition assay for in vitro B. caballi growth inhibition studies
Biochem/physiol Actions
Features and Benefits
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
We offer agonists, antagonists, modulators and other bioactive small molecules for immune system signaling target identification and validation, as well as a variety of antibiotics, antivirals, and antifungals.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service