H6886
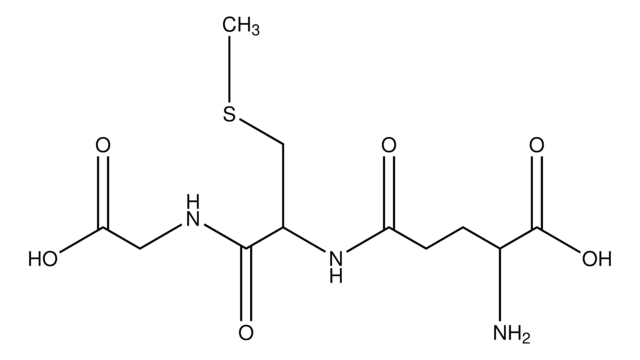
S-Hexylglutathione
>98% (TLC), suitable for ligand binding assays
Synonym(s):
S-Hexyl-L-glutathione reduced
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C16H29N3O6S
CAS Number:
Molecular Weight:
391.48
Beilstein:
5629635
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
S-Hexylglutathione,
Assay
>98% (TLC)
Quality Level
form
powder
technique(s)
ligand binding assay: suitable
mp
200-202 °C
storage temp.
2-8°C
SMILES string
CCCCCCSC[C@H](NC(=O)CC[C@H](N)C(O)=O)C(=O)NCC(O)=O
InChI
1S/C16H29N3O6S/c1-2-3-4-5-8-26-10-12(15(23)18-9-14(21)22)19-13(20)7-6-11(17)16(24)25/h11-12H,2-10,17H2,1H3,(H,18,23)(H,19,20)(H,21,22)(H,24,25)/t11-,12-/m0/s1
InChI key
HXJDWCWJDCOHDG-RYUDHWBXSA-N
Related Categories
Amino Acid Sequence
S-Hexyl-Glu-Cys-Gly
Application
Ligand useful for affinity chromatography of glutathione-S-transferase and glutathione peroxidase.
Biochem/physiol Actions
S-Hexylglutathione may be used as an affinity chromatography ligand of glutathione-S-transferase and glutathione peroxidase. S-Hexylglutathione is also used as an inhibitor to study the specificity and kinetics of enzymes such as mitochondrial membrane-bound glutathione transferase(s) (mtMGST1).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Natalya Kurochkina et al.
Journal of theoretical biology, 283(1), 92-102 (2011-05-31)
Helix-helix parallel interfaces can be characterized by certain combinations of amino acids, which repeatedly occur at core positions a and d (leucine zipper nomenclature) in homologous and nonhomologous proteins and influence interhelical angles. Applied for the prediction of interhelical angles
Y Hathout et al.
Chemical research in toxicology, 9(6), 1044-1049 (1996-09-01)
Interaction of chlorambucil and the glutathione-depleted human placenta pi class glutathione S-transferase (pi GST) results in the formation of a complex between the drug and the protein at physiological pH. This complex is not formed in the presence of glutathione
Liqing Chen et al.
Acta crystallographica. Section D, Biological crystallography, 59(Pt 12), 2211-2217 (2003-12-04)
Glutathione S-transferases (GSTs) are a major family of detoxification enzymes which possess a wide range of substrate specificities. Most organisms possess many GSTs belonging to multiple classes. Interest in GSTs in insects is focused on their role in insecticide resistance;
Markus Perbandt et al.
The Journal of biological chemistry, 280(13), 12630-12636 (2005-01-11)
Onchocerciasis is a debilitating parasitic disease caused by the filarial worm Onchocerca volvulus. Similar to other helminth parasites, O. volvulus is capable of evading the host's immune responses by a variety of defense mechanisms, including the detoxification activities of the
Emilee E Colón-Lorenzo et al.
Frontiers in pharmacology, 11, 246-246 (2020-04-08)
Plasmodium falciparum parasites are increasingly drug-resistant, requiring the search for novel antimalarials with distinct modes of action. Enzymes in the glutathione pathway, including glutathione S-transferase (GST), show promise as novel antimalarial targets. This study aims to better understand the biological
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service