G7882
L-Glutamic Dehydrogenase from bovine liver
Type III, lyophilized powder, ≥20 units/mg protein
Synonym(s):
L-GLDH, L-Glutamate:NAD[P]+ Oxidoreductase (deaminating), Glutamate Dehydrogenase from bovine liver
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Recommended Products
biological source
bovine liver
Quality Level
type
Type III
form
lyophilized powder
specific activity
≥20 units/mg protein
UniProt accession no.
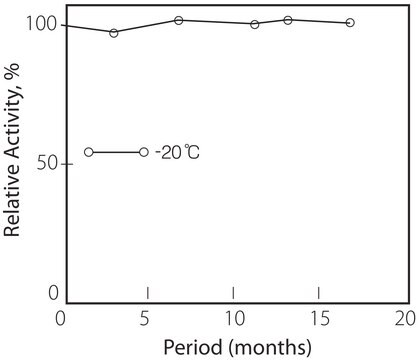
storage temp.
−20°C
Gene Information
cow ... GLUD1(281785)
Looking for similar products? Visit Product Comparison Guide
Application
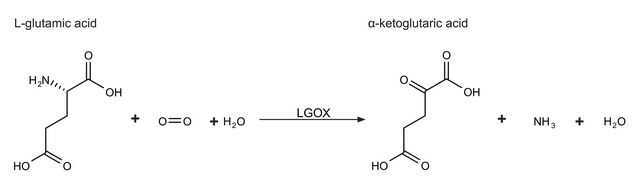
L-Glutamic Dehydrogenase was used to catalyzes the conversion of isocitrate into a-ketoglutarate and carbon dioxide.
Biochem/physiol Actions
Mammalian forms of this enzyme, including this bovine form, can use either NADP(H) or NAD(H) as coenzymes. L-glutamic dehydrogenase plays a unique role in mammalian metabolism. The reverse reaction catalyzed by this enzyme is the only pathway by which ammonia can become bound to the α-carbon atom of an α-carboxylic acid and thus, is the only source of de novo amino acid synthesis in mammalian species.
The bovine enzyme is characterized by three sets of properties:
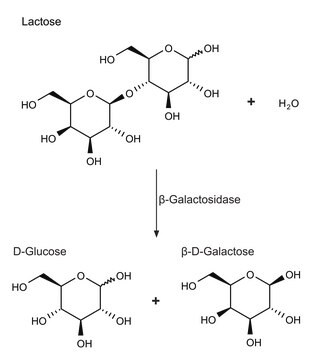
L-glutamic dehydrogenase catalyzes the conversion of glutamate to α-ketoglutarate.
The bovine enzyme is characterized by three sets of properties:
- It has a reversible concentration-dependent association, producing higher molecular weight forms.
- Forms tight enzyme-reduced coenzyme-substrate ternary complexes whose rates of dissociation modulate the steady-state reaction rates.
- Exhibits a wide variety of effects from the binding of any of a number of nucleotide modifiers.
L-glutamic dehydrogenase catalyzes the conversion of glutamate to α-ketoglutarate.
Packaging
Package size based on protein content
Unit Definition
One unit will reduce 1.0 μmole of α-ketoglutarate to L-glutamate per min at pH 7.3 at 25 °C, in the presence of ammonium ions.
Physical form
Contains citrate and potassium phoshate buffer salts.
Analysis Note
Protein determined by biuret
substrate
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Decreased carbohydrate metabolism enzyme activities in the glaucomatous trabecular meshwork
Junk AK, Goel M, Mundorf T, Rockwood EJ, Bhattacharya SK
Molecular Vision, 10, 1286-1291 (2010)
Roy M Daniel et al.
The Biochemical journal, 425(2), 353-360 (2009-10-24)
Experimental data show that the effect of temperature on enzymes cannot be adequately explained in terms of a two-state model based on increases in activity and denaturation. The Equilibrium Model provides a quantitative explanation of enzyme thermal behaviour under reaction
Laszlo Tretter et al.
Journal of neurochemistry, 83(4), 855-862 (2002-11-08)
Previously we have reported that oxidative stress induced by hydrogen peroxide exacerbates the effect of an Na+ load in isolated nerve terminals, with a consequence of an ATP depletion, [Ca2+]i and [Na+]i deregulation, and collapse of mitochondrial membrane potential. In
K E Snider et al.
The Journal of clinical endocrinology and metabolism, 98(2), E355-E363 (2013-01-01)
Hypoglycemia due to congenital hyperinsulinism (HI) is caused by mutations in 9 genes. Our objective was to correlate genotype with phenotype in 417 children with HI. Mutation analysis was carried out for the ATP-sensitive potassium (KATP) channel genes (ABCC8 and
Johannes Heidemann et al.
International journal of mass spectrometry, 447, 116240-116240 (2020-11-28)
As a fundament in many biologically relevant processes, endocytosis in its different guises has been arousing interest for decades and still does so. This is true for the actual transport and its initiation alike. In clathrin-mediated endocytosis, a comparatively well
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service