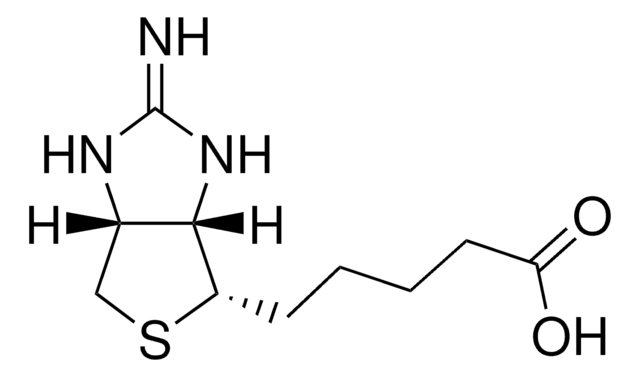
D1411
d-Desthiobiotin
≥98% (TLC)
Synonym(s):
5-Methyl-2-oxo-4-imidazolidinehexanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C10H18N2O3
CAS Number:
Molecular Weight:
214.26
EC Number:
MDL number:
UNSPSC Code:
12352200
eCl@ss:
34058011
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥98% (TLC)
form
powder
storage temp.
2-8°C
SMILES string
C[C@@H]1NC(=O)N[C@@H]1CCCCCC(O)=O
InChI
1S/C10H18N2O3/c1-7-8(12-10(15)11-7)5-3-2-4-6-9(13)14/h7-8H,2-6H2,1H3,(H,13,14)(H2,11,12,15)/t7-,8+/m0/s1
InChI key
AUTOLBMXDDTRRT-JGVFFNPUSA-N
Application
d-Desthiobiotin has been used as a component of elution buffers:
- to elute the precipitated proteins from the resin during coimmunoprecipitation
- to elute human Cdc45–MCM–GINS (CMG) helicase during chromatographic protein purification
- to elute the heterodimeric kinesin family member 3A/B (KIF3A/B) and kinesin associated protein 3 (KAP3)-binding APC armadillo (APCARM) domain during chromatographic purification
d-Desthiobiotin is used in affinity chromatography and protein chromatography. d-Desthiobiotin can be used for protein labeling, detection and isolation.
Biochem/physiol Actions
Desthiobiotin is a non-sulfur containing biotin derivative that binds less strongly to biotin-binding proteins and is easily dislocated by biotin.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Teegan A Delli-Bovi et al.
BMC biotechnology, 10, 73-73 (2010-10-13)
Biotin is an essential enzyme cofactor that acts as a CO2 carrier in carboxylation and decarboxylation reactions. The E. coli genome encodes a biosynthetic pathway that produces biotin from pimeloyl-CoA in four enzymatic steps. The final step, insertion of sulfur
Bi-Huang Hu et al.
Analytical chemistry, 79(19), 7275-7285 (2007-08-24)
We describe a new method for encoded synthesis, efficient on-resin screening, and rapid unambiguous sequencing of combinatorial peptide libraries. An improved binary tag system for encoding peptide libraries during synthesis was designed to facilitate unequivocal assignment of isobaric residues by
Rocio Rodriguez-Melendez et al.
The Journal of nutrition, 133(5), 1259-1264 (2003-05-06)
In mammals, biotin serves as a coenzyme for carboxylases such as propionyl-CoA carboxylase. The expression of genes encoding interleukin-2 (IL-2) and IL-2 receptor (IL-2R)gamma also depends on biotin. Biotin metabolites are structurally similar to biotin, and their concentrations in tissues
M Sárdy et al.
Clinical chemistry, 45(12), 2142-2149 (1999-12-10)
Tissue transglutaminase (TGc) has recently been identified as the major, if not the sole, autoantigen of gluten-sensitive enteropathy (GSE). We developed and validated an ELISA based on the human recombinant antigen and compared it to existing serological tests for GSE
Sudha Purushothaman et al.
PloS one, 3(5), e2320-e2320 (2008-05-30)
Fatty acids are indispensable constituents of mycolic acids that impart toughness & permeability barrier to the cell envelope of M. tuberculosis. Biotin is an essential co-factor for acetyl-CoA carboxylase (ACC) the enzyme involved in the synthesis of malonyl-CoA, a committed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service