All Photos(4)
About This Item
Empirical Formula (Hill Notation):
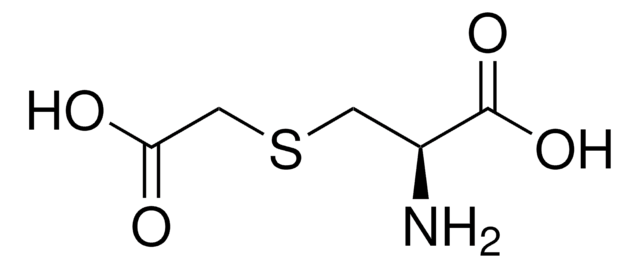
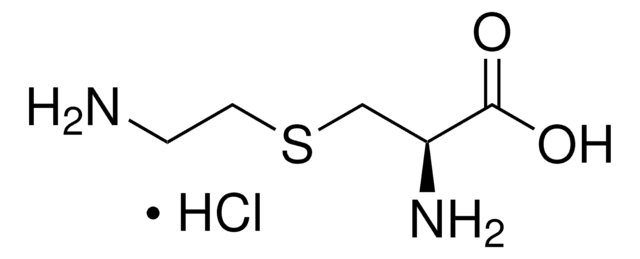
C5H9NO4S
CAS Number:
Molecular Weight:
179.19
Beilstein:
1725012
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Assay
>98% (TLC)
Quality Level
form
powder
technique(s)
cell culture | mammalian: suitable
color
white
mp
200 °C
storage temp.
2-8°C
SMILES string
N[C@@H](CSCC(O)=O)C(O)=O
InChI
1S/C5H9NO4S/c6-3(5(9)10)1-11-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1
InChI key
GBFLZEXEOZUWRN-VKHMYHEASA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Biochem/physiol Actions
S-Carboxymethyl-L-cysteine is studied as a small molecule mucoactive drug in vivo. These studies include analyzing the oxidative metabolism of S-carboxymethyl-L-cysteine by enzymes such as phenylalanine monooxygenase(s).
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Recombinant heteromeric phenylalanine monooxygenase and the oxygenation of carbon and sulfur substrates.
Boonyapiwat B, Mitchell SC, et al.
J. Pharm. Pharm. Sci., 63, 558-564 (2011)
Human phenylalanine monooxygenase and thioether metabolism.
Boonyapiwat B, Panaretou B, et al.
J. Pharm. Pharm. Sci., 61, 63-67 (2009)
Marty K Soehnlen et al.
The Journal of antimicrobial chemotherapy, 66(3), 574-577 (2011-03-12)
To screen novel small molecule compounds for inhibition of Mycoplasma bovis growth and to characterize their activity in terms of dose-dependency and ability to function in milk. Using a tetrazolium salt cytotoxicity assay, 480 natural compounds were screened to determine
Panayotis Panagopoulos et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 39(4), 219-223 (2009-12-29)
The aim of this study was to investigate the feasibility of employing S-carboxymethyl-L-cysteine as a treatment of chronic obstructive pulmonary disease in dogs. To this end the pharmacokinetic parameters of orally administered S-carboxymethyl-L-cysteine were determined in the dog, cow and
Masanori Asada et al.
Respiratory physiology & neurobiology, 180(1), 112-118 (2011-11-15)
To examine the effects of l-carbocisteine on airway infection with respiratory syncytial (RS) virus, human tracheal epithelial cells were pretreated with l-carbocisteine and infected with RS virus. Viral titer, virus RNA, and pro-inflammatory cytokine secretion, including interleukin (IL)-1 and IL-6
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service