All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H18N4O2S
CAS Number:
Molecular Weight:
258.34
Beilstein:
28347
MDL number:
UNSPSC Code:
12352203
PubChem Substance ID:
NACRES:
NA.46
Recommended Products
Quality Level
Assay
≥97% (TLC)
form
powder
solubility
DMSO: ≤20 mg/mL
storage temp.
2-8°C
SMILES string
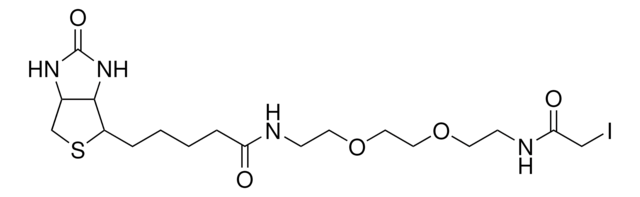
[H][C@]12CS[C@@H](CCCCC(=O)NN)[C@@]1([H])NC(=O)N2
InChI
1S/C10H18N4O2S/c11-14-8(15)4-2-1-3-7-9-6(5-17-7)12-10(16)13-9/h6-7,9H,1-5,11H2,(H,14,15)(H2,12,13,16)/t6-,7-,9-/m0/s1
InChI key
KOZWHQPRAOJMBN-ZKWXMUAHSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Biotin hydrazide is a biotinylation reagent used to biotinylate glycoproteins with their sugar moieties. Biotin hydrazide can be used to prepare biotin-conjugated alginate. It can also be used for covalent attachment to PAAc via carbodi-imide cross linking.
Application
(+)-Biotin hydrazide has been used:
- the modification of alginate
- for the labelling of mitochondrial proteins from non-muscle tissues
- as a component of glycoprotein staining solution
- in periodic acid-biotin-hydrazide (PABH) assay for mucins
- for labeling surface functional groups, biologically active molecules such as antibodies, lectins, sugars, nucleic acids or molecules with free carboxylic or keto groups.
- for coupling to glycoproteins through the carbohydrate by hydrazone formation
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Suppressive Effect of the o-Amylase Inhibitor Albumin from Buckwheat (Fagopyrum esculentum Moench) on Postprandial Hyperglycaemia
Impact of Bioactive Peptides on Human Health, 157-157 (2016)
E P Diamandis et al.
Clinical chemistry, 37(5), 625-636 (1991-05-01)
The biotin-(strept)avidin system has been used for many years in a variety of different applications. Here we present a general overview of the system, describe its components and advantages, and show how the system is used in various applications, with
Emma Luong-Van et al.
Biointerphases, 4(2), 13-18 (2010-04-23)
The spatial control of cells on a surface and the patterning of multiple cell types is an important tool for fundamental biological research and tissue engineering applications. A novel technique is described for the controlled seeding of multiple cell types
Enhanced capture and release of circulating tumor cells using hollow glass microspheres with a nanostructured surface
Dong Z, et al.
Nanoscale, 10(35), 16795-16804 (2018)
Ajay M Shah et al.
Analytical chemistry, 84(8), 3682-3688 (2012-03-15)
Microfluidic systems for affinity-based cell isolation have emerged as a promising approach for the isolation of specific cells from complex matrices (i.e., circulating tumor cells in whole blood). However, these technologies remain limited by the lack of reliable methods for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service