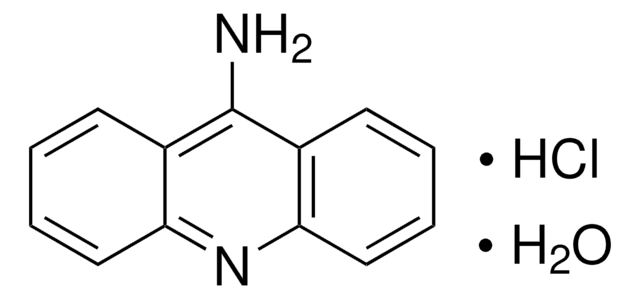
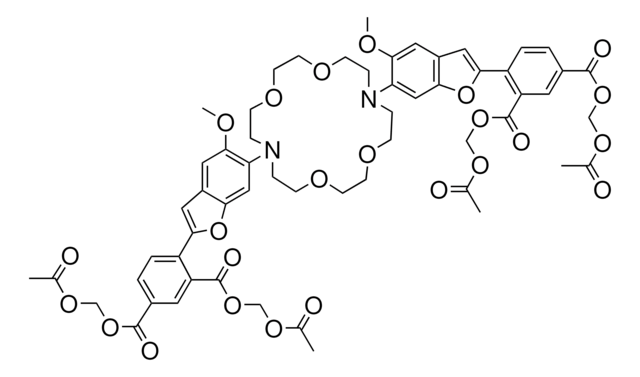
14562
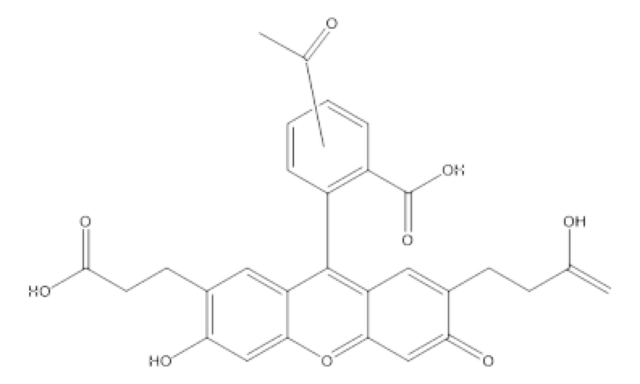
2′,7′-Bis(2-carboxyethyl)-5(6)-carboxyfluorescein tetrakis(acetoxymethyl) ester
BioReagent, for fluorescence
Synonym(s):
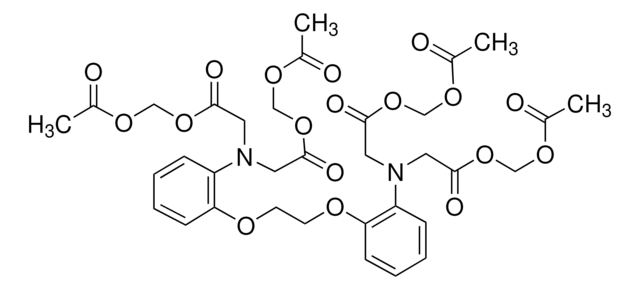
BCECF-AM
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C39H36O19
Molecular Weight:
808.69
MDL number:
UNSPSC Code:
12352108
NACRES:
NA.32
Recommended Products
grade
for fluorescence
Quality Level
product line
BioReagent
form
solid
solubility
DMF: soluble
DMSO: soluble
acetonitrile: soluble
fluorescence
λex 482 nm; λem 528 nm in 0.1 M Tris pH 8.0 (esterase)
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Application
An uncharged, nonfluorescent molecule that is useful for noninvasive bulk loading of cell suspension because it is capable of permeating the cell membrane. Cellular nonspecific esterases cleave its lipophilic blocking groups, resulting in a charged, fluorescent form of the molecule. Compared to the parent compound, this charged derivative leaks out of the cell at a much slower rate. Utilized for pH measurements in perfused tissues, intercellular spaces, mammalian cells, plant cells, bacteria, and yeast. Also, employed to investigate in assays for cellular functional properties such as adhesion, multi-drug resistance, viability and cytotoxicity, apoptosis and chemotaxis.
Cell-permeable ester of BCECF that, on hydrolysis by cytosolic esterases, yields the intracellularly trapped pH-indicator BCECF. Allows simultaneous recording of cell volume changes and intracellular pH in single osteosarcoma cells.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
L Homolya et al.
The Journal of biological chemistry, 268(29), 21493-21496 (1993-10-15)
In this report we show that NIH-3T3 mouse fibroblasts stably expressing the human multidrug transporter (MDR1 or P-glycoprotein), in contrast to the control NIH-3T3 cells, actively extrude the hydrophobic acetoxymethyl ester (AM) derivatives used for cellular loading of various fluorescent
J S Leeder et al.
Analytical biochemistry, 177(2), 364-372 (1989-03-01)
Studies of drug toxicity, toxicologic structure-function relationships, screening of idiosyncratic drug reactions, and a variety of cytotoxic events and cellular functions in immunology and cell biology require the sensitive and rapid processing of often large numbers of cell samples. This
J T Seo et al.
Pflugers Archiv : European journal of physiology, 426(1-2), 75-82 (1994-01-01)
Intracellular pH (pHi) has been measured in intact, perfused rat mandibular salivary glands loaded with the fluorescent pH indicator BCECF [2',7'-bis(2-carboxyethyl)-5(6)- carboxyfluorescein]. Glands mounted in the cuvette of a conventional bench-top spectro-fluorometer were perfused for 5 min with the acetoxymethyl
G R Martin et al.
Cancer research, 54(21), 5670-5674 (1994-11-01)
The tumor interstitial pH and its modification play a significant role in cancer treatment. Current in vivo pH measurement techniques are invasive and/or provide poor spatial resolution. Therefore, there are no data on perivascular interstitial pH gradients in normal or
S Goelz et al.
The Journal of biological chemistry, 269(2), 1033-1040 (1994-01-14)
The mammalian cDNA encoding alpha (1,3)-fucosyltransferase (alpha (1,3)Fuc-T) termed ELAM-1 ligand fucosyltransferase (ELFT) or Fuc-TIV was previously cloned by three groups who reported different results from transfection studies Goelz et al. (Goelz, S. E., Hession, C., Goff, D., Griffiths, B.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service