T6508
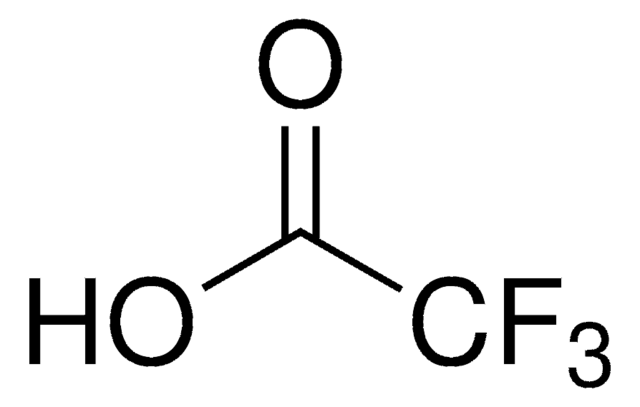
Trifluoroacetic acid
ReagentPlus®, 99%
Synonym(s):
TFA
About This Item
Recommended Products
vapor density
3.9 (vs air)
Quality Level
vapor pressure
97.5 mmHg ( 20 °C)
product line
ReagentPlus®
Assay
99%
form
liquid
impurities
≤0.05% water
refractive index
n20/D 1.3 (lit.)
pH
1 (10 g/L)
bp
72.4 °C (lit.)
mp
−15.4 °C (lit.)
solubility
ethanol: soluble 0.33 mL/mL
density
1.489 g/mL at 20 °C (lit.)
SMILES string
OC(C(F)(F)F)=O
InChI
1S/C2HF3O2/c3-2(4,5)1(6)7/h(H,6,7)
InChI key
DTQVDTLACAAQTR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- For the cleavage of nitrogen and oxygen protecting groups such as N-Boc, N-benzyloxymethyl, benzyl ether, p-methoxybenzyl ether, t-butyl ether, t-butyloxymethyl ether, triphenylmethyl ether, and dimethyl acetals.
- In the Baeyer–Villiger oxidation reactions in combination with sodium percarbonate.,·
- For the C-H trifluoromethylation of arenes.
TFA can also be used as:
- A solvent in atom transfer cyclization reactions and polymer processes.
- A catalyst in the synthesis of ε-caprolactam via Beckmann rearrangement of cyclohexanone oxime in aprotic solvents.
Packaging
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1A
Storage Class Code
8A - Combustible, corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
212.0 °F - Pensky-Martens closed cup
Flash Point(C)
> 100 °C - Pensky-Martens closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Fmoc resin cleavage and deprotection follows the difficult task of detaching the peptide from the resin support and removing all the side-chain protecting groups of the amino acid residues to yield the desired peptide.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service