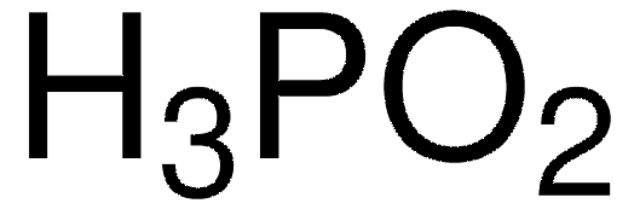215112
Phosphorous acid
99%
Synonym(s):
Phosphonic acid
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Empirical Formula (Hill Notation):
H3O3P
CAS Number:
Molecular Weight:
82.00
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
Assay
99%
form
crystals or chunks
flakes
solubility
water: soluble
density
1.651 g/mL at 25 °C (lit.)
SMILES string
[H]P(O)(O)=O
InChI
1S/H3O3P/c1-4(2)3/h4H,(H2,1,2,3)
InChI key
ABLZXFCXXLZCGV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Phosphorous acid (or phosphonic acid) is a phosphorus oxoacid commonly used as an intermediate in chemical synthesis for the production of various phosphorus compounds. It is also used as a strong reducing agent in organic synthesis.
Application
Phosphorous acid (H3PO3, orthophosphorous acid) may be used as one of the reaction components for the synthesis of the following:
- α-aminomethylphosphonic acids via Mannich-Type Multicomponent Reaction
- 1-aminoalkanephosphonic acids via amidoalkylation followed by hydrolysis
- N-protected α-aminophosphonic acids (phospho-isosteres of natural amino acids) via amidoalkylation reaction
Signal Word
Danger
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1A
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Amidoalkylation of phosphorous acid.
Vinyukov AV, et al.
Russ. J. Gen. Chem., 85(2), 366-369 (2015)
Review on modern advances of chemical methods for the introduction of a phosphonic acid group.
Charles S Demmer et al.
Chemical reviews, 111(12), 7981-8006 (2011-10-21)
Marco Esposito et al.
European journal of oral implantology, 6(3), 227-236 (2013-11-02)
To evaluate the safety and clinical efficacy of a novel surface treatment (SurfLink®, Nano Bridging Molecules, Gland, Switzerland) on titanium dental implants. SurfLink consists of a monolayer of permanently bound multi-phosphonic acid molecules, which mimics the surface of naturally occurring
Abraham Vega et al.
Langmuir : the ACS journal of surfaces and colloids, 28(21), 8046-8051 (2012-05-05)
Phosphonic acid monolayers are being considered as versatile surface modification agents due to their unique ability to attach to surfaces in different configurations, including mono-, bi-, or even tridentate arrangements. Tethering by aggregation and growth (T-BAG) of octadecylphosphonic acid (ODPA)
Amidoalkylation of phosphorous acid.
Oleksyszyn J and Gruszeckae.
Tetsu to Hagane, 22(36), 3537-3540 (1981)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service