8.55068
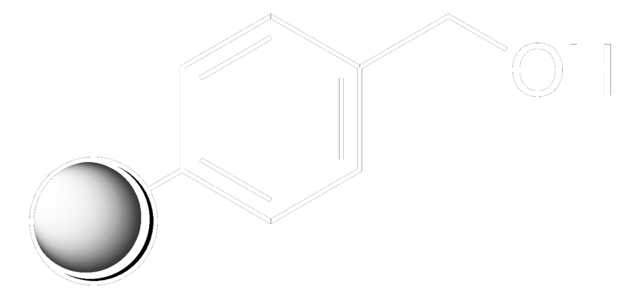
Hydroxymethyl polystyrene
100-200 mesh, 1% DVB, Novabiochem®
Synonym(s):
Polystyrene-CH 2OH (100-200 mesh)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
13111023
NACRES:
NA.22
Recommended Products
Product Name
Hydroxymethyl polystyrene (100-200 mesh), 1% DVB, Novabiochem®
Quality Level
product line
Novabiochem®
form
beads
reaction suitability
reaction type: Boc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
alcohol
storage temp.
2-30°C
Related Categories
General description
This resin is ideal for the solid phase immobilization of carboxylic acids. Less cross-linking is observed than with Merrifield resin during the attachment of diacids [1]. Reaction with a suitable phosgene equivalent converts this resin to a support suitable for the immobilization of amines [2]. Release of carboxylic acids from this support is normally effected by treatment of the resin with H For TFMSA, or by hydrogenolysis [3]. Alcohols can be liberated by reduction with DIBAL [4] or LiBH4[5]. Methyl esters can be produced by transesterification with MeONa [6] or Ti(OEt)4/CH3CO2Me [7]. Carboxamides are also accessible via Lewis acid catalyzed aminolysis [8].
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis Resins
Literature references
[1] J. M. Goldwasser, et al. (1978) Can. J. Chem., 56,1562.
[2] D. J. Burdick, et al. (1993) Tetrahedron Lett., 34,2589.
[3] J. M. Schlatter, et al. (1977) Tetrahedron Lett., 2851.
[4] M. J. Kurth, et al. (1994) J. Org. Chem., 59,5862.
[5] J. M. Stewart & J. D. Young in ′Solid Phase Peptide Synthesis, 2nd Ed.′, Rockford, Illinois, Pierce Chemical Company, 1984, pp. 92.
[6] R. Frenette, et al. (1994) Tetrahedron Lett., 35,9177.
[7] L. T. Tietze & A. Steinmetz (1996) Angew. Chem. Int. Ed.Engl., 35, 651.
[8] D. R. Barn, et al. (1996) TetrahedronLett., 37, 3213.
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis Resins
Literature references
[1] J. M. Goldwasser, et al. (1978) Can. J. Chem., 56,1562.
[2] D. J. Burdick, et al. (1993) Tetrahedron Lett., 34,2589.
[3] J. M. Schlatter, et al. (1977) Tetrahedron Lett., 2851.
[4] M. J. Kurth, et al. (1994) J. Org. Chem., 59,5862.
[5] J. M. Stewart & J. D. Young in ′Solid Phase Peptide Synthesis, 2nd Ed.′, Rockford, Illinois, Pierce Chemical Company, 1984, pp. 92.
[6] R. Frenette, et al. (1994) Tetrahedron Lett., 35,9177.
[7] L. T. Tietze & A. Steinmetz (1996) Angew. Chem. Int. Ed.Engl., 35, 651.
[8] D. R. Barn, et al. (1996) TetrahedronLett., 37, 3213.
Linkage
Replaces: 01-64-0110
Analysis Note
Color (visual): white to yellow to beige
Appearance of substance (visual): beads
Loading (determined from the substitution of the Fmoc-Leu loaded resin): 0.60 - 1.60 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene - 1% DVB) 100 -200 mesh
Appearance of substance (visual): beads
Loading (determined from the substitution of the Fmoc-Leu loaded resin): 0.60 - 1.60 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene - 1% DVB) 100 -200 mesh
Legal Information
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service