1.06146
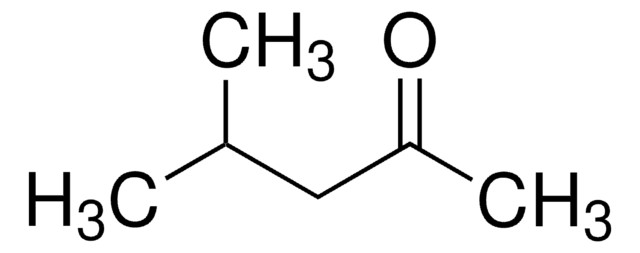
Isobutyl methyl ketone
for extraction analysis EMSURE® ACS,Reag. Ph Eur
Synonym(s):
4-Methyl-2-pentanone, Isobutyl methyl ketone, Isopropylacetone, MIBK, Methyl isobutyl ketone
About This Item
Recommended Products
grade
ACS reagent
Quality Level
Agency
reag. Ph. Eur.
vapor density
3.5 (vs air)
product line
EMSURE®
Assay
≥99% (GC)
form
liquid
autoignition temp.
840 °F
potency
2080 mg/kg LD50, oral (Rat)
>16000 mg/kg LD50, skin (Rabbit)
expl. lim.
1.2-8 %, 93 °F
technique(s)
UV/Vis spectroscopy: suitable
impurities
≤0.00005% Al (Aluminium)
≤0.001 meq/g Alkalinity
≤0.002 meq/g Titrable acid
≤0.1% Water
evapn. residue
≤0.0010%
color
clearAPHA: ≤10
refractive index
n20/D 1.395 (lit.)
pH
7 (20 °C)
bp
117-118 °C
mp
−80 °C (lit.)
transition temp
flash point 14 °C
density
0.801 g/mL at 25 °C (lit.)
cation traces
B: ≤0.000002%
Ba: ≤0.00001%
Ca: ≤0.00005%
Cd: ≤0.000005%
Co: ≤0.000002%
Cr: ≤0.000002%
Cu: ≤0.000002%
Fe: ≤0.00001%
Mg: ≤0.00001%
Mn: ≤0.000002%
Ni: ≤0.000002%
Pb: ≤0.00001%
Sn: ≤0.00001%
Zn: ≤0.00001%
storage temp.
2-30°C
SMILES string
CC(C)CC(C)=O
InChI
1S/C6H12O/c1-5(2)4-6(3)7/h5H,4H2,1-3H3
InChI key
NTIZESTWPVYFNL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Determination of cadmium in biological samples by inductively coupled plasma atomic emission spectrometry after extraction with 1,5-bis(di-2-pyridylmethylene) thiocarbonohydrazide.: This study utilizes Isobutyl methyl ketone (IBMK) as a solvent in the extraction process for determining cadmium in biological samples. The research highlights the efficacy of IBMK in enhancing the sensitivity and accuracy of inductively coupled plasma atomic emission spectrometry (ICP-AES), crucial for trace metal analysis in various biological and environmental samples (Espinosa Almendro et al., 1992).
- Colorimetric determination of acetylenic hypnotics by formation of silver acetylides.: This research demonstrates a colorimetric method for determining acetylenic hypnotics using Isobutyl methyl ketone (IBMK) as a solvent. IBMK is instrumental in the formation of silver acetylides, facilitating the detection and quantification of these compounds in pharmaceutical formulations. The study underscores the versatility of IBMK in analytical chemistry applications, particularly in the pharmaceutical industry (Rizk et al., 1980).
Analysis Note
Identity (IR): conforms
Appearance: clear
Color: ≤ 10 Hazen
Titrable acid: ≤ 0.002 meq/g
Alkalinity: ≤ 0.001 meq/g
Density (d 20 °C/20 °C): 0.799 - 0.805
Boiling point: 114 - 117 °C
Distilling range: ≤ 4 °C
Al (Aluminium): ≤ 0.00005 %
B (Boron): ≤ 0.000002 %
Ba (Barium): ≤ 0.00001 %
Ca (Calcium): ≤ 0.00005 %
Cd (Cadmium): ≤ 0.000005 %
Co (Cobalt): ≤ 0.000002 %
Cr (Chromium): ≤ 0.000002 %
Cu (Copper): ≤ 0.000002 %
Fe (Iron): ≤ 0.00001 %
Mg (Magnesium): ≤ 0.00001 %
Mn (Manganese): ≤ 0.000002 %
Ni (Nickel): ≤ 0.000002 %
Pb (Lead): ≤ 0.00001 %
Sn (Tin): ≤ 0.00001 %
Zn (Zinc): ≤ 0.00001 %
Evaporation residue: ≤ 0.0010 %
Water: ≤ 0.1 %
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Carc. 2 Inhalation - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Target Organs
Central nervous system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
57.2 °F - closed cup
Flash Point(C)
14 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service