All Photos(3)
About This Item
Empirical Formula (Hill Notation):
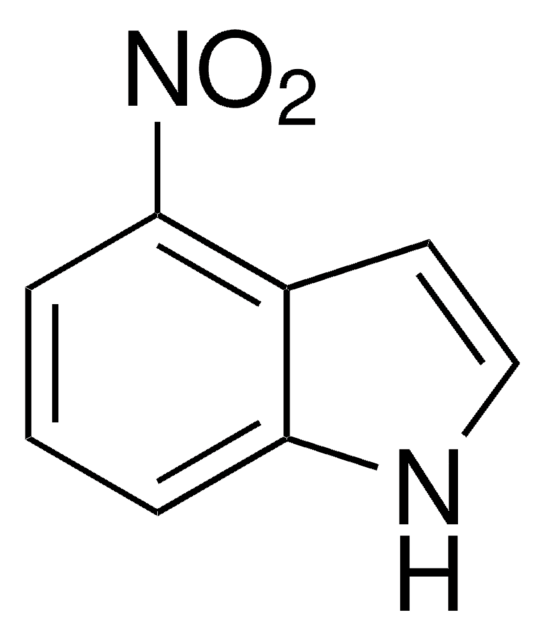
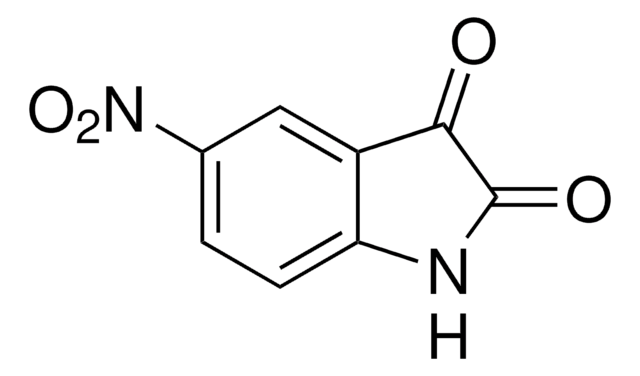
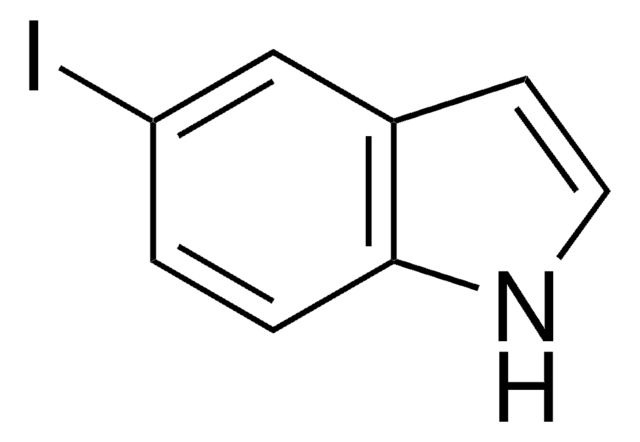
C8H6N2O2
CAS Number:
Molecular Weight:
162.15
Beilstein:
383777
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
140-142 °C (lit.)
SMILES string
[O-][N+](=O)c1ccc2[nH]ccc2c1
InChI
1S/C8H6N2O2/c11-10(12)7-1-2-8-6(5-7)3-4-9-8/h1-5,9H
InChI key
OZFPSOBLQZPIAV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for preparation of:
- Pharmaceutically active 2-oxo-1-pyrrolidine analogues
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Protein Kinase Inhibitors and antiproliferative agents
- Positive Allosteric Modulators of Metabotropic Glutamate Receptor 4 (mGlu4)
- Antifungal agents
- Cannabinoid receptor type 1 (CB1) antagonists
- Potential anticancer agents
- Potential antivascular agents
- Selective Anti-leukemic agents
- Anti human immunodeficiency virus subtype 1 (HIV-1) agents
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
José Gallego et al.
Nucleic acids research, 35(9), 2904-2912 (2007-04-18)
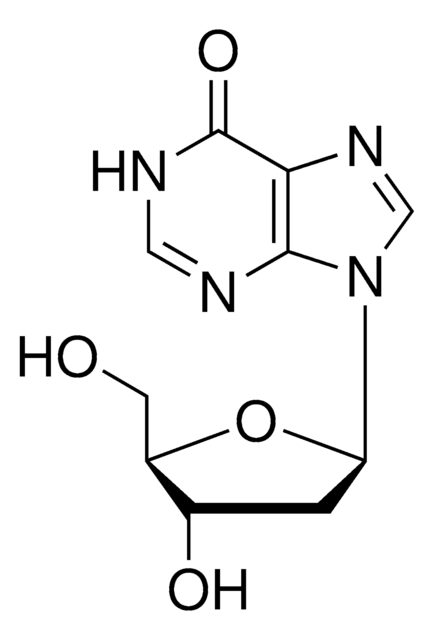
Universal bases hybridize with all other natural DNA or RNA bases, and have applications in PCR and sequencing. We have analysed by nuclear magnetic resonance spectroscopy the structure and dynamics of three DNA oligonucleotides containing the universal base analogues 5-nitroindole
H Challa et al.
Organic letters, 1(10), 1639-1641 (2000-06-03)
[formula: see text] The syntheses of PNA oligomers containing potential ambiguous nucleobase analogues, namely 3-nitropyrrole and 5-nitroindole, have been accomplished. Hybridization properties of these PNAs with complementary oligodeoxynucleotides were evaluated by thermal denaturation experiments. Both novel residues exhibited little variation
P M Vallone et al.
Nucleic acids research, 27(17), 3589-3596 (1999-08-14)
Effects of the universal base 5-nitroindole on the thermodynamic stability of DNA hairpins having a 6 bp stem and four base loops were investigated by optical absorbance and differential scanning calorimetry techniques. Melting studies were conducted in buffer containing 115
Use of 5-nitroindole-2'-deoxyribose-5'-triphosphate for labelling and detection of oligonucleotides.
C L Smith et al.
Nucleosides & nucleotides, 17(1-3), 555-564 (1998-08-26)
The 5'-triphosphate of 5-nitroindole-2'-deoxyriboside has been shown to be a good substrate for terminal deoxynucleotidyl transferase (TdT). An antibody has been prepared for the detection of 5-nitroindole and has been used for the detection of 5-nitroindole tailed DNA both in
David Loakes et al.
Journal of the American Chemical Society, 131(41), 14827-14837 (2009-09-26)
Hydrophobic base analogues (HBAs) have shown great promise for the expansion of the chemical and coding potential of nucleic acids but are generally poor polymerase substrates. While extensive synthetic efforts have yielded examples of HBAs with favorable substrate properties, their
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service