D32202
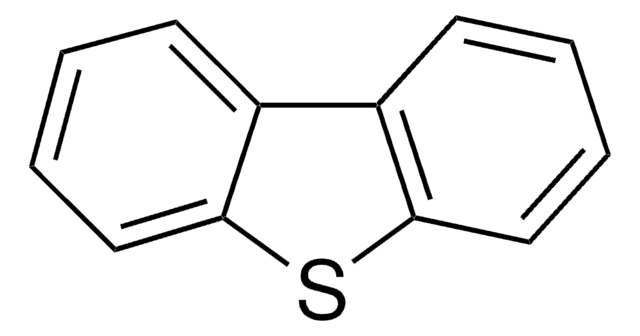
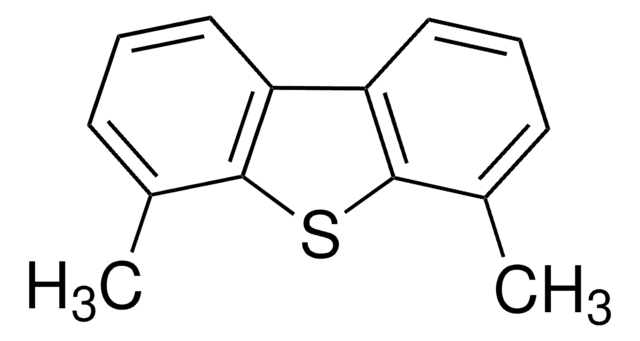
Dibenzothiophene
98%
Synonym(s):
DBT, Diphenylene sulfide
About This Item
Recommended Products
Quality Level
Assay
98%
form
powder or crystals
bp
332-333 °C (lit.)
mp
97-100 °C (lit.)
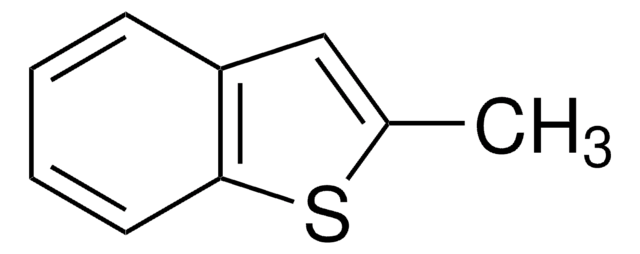
SMILES string
c1ccc2c(c1)sc3ccccc23
InChI
1S/C12H8S/c1-3-7-11-9(5-1)10-6-2-4-8-12(10)13-11/h1-8H
InChI key
IYYZUPMFVPLQIF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- A starting material for the synthesis of corresponding sulfoxide and sulfone by oxidative desulfurization using various catalysts.
- A template for the synthesis of surface molecular imprinted polymer (SMIP). SMIP is applicable for the removal of dibenzothiophene during desulfurization of the gasoline
- A precursor for the synthesis of DBT based π-conjugating polymers.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
338.0 °F
Flash Point(C)
170 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
GC Analysis of Polynuclear Aromatic Hydrocarbons (PAHs) in Salmon on SPB®-608 (20 m x 0.18 mm I.D., 0.18 µm) after QuEChERS Cleanup using Supel™ QuE Z-Sep, Fast GC Analysis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service