All Photos(2)
About This Item
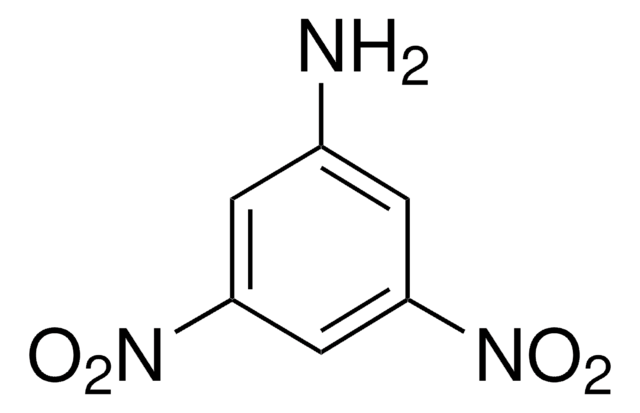
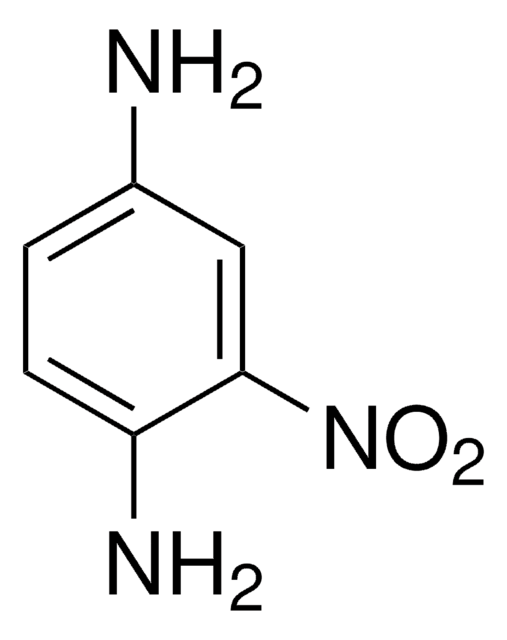
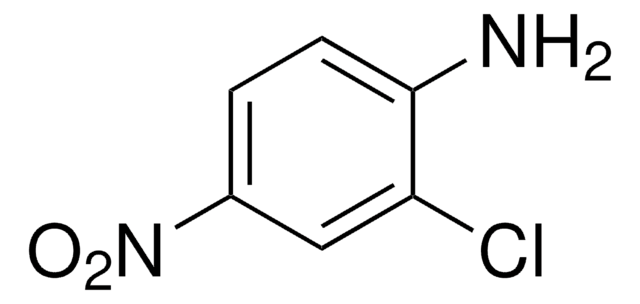
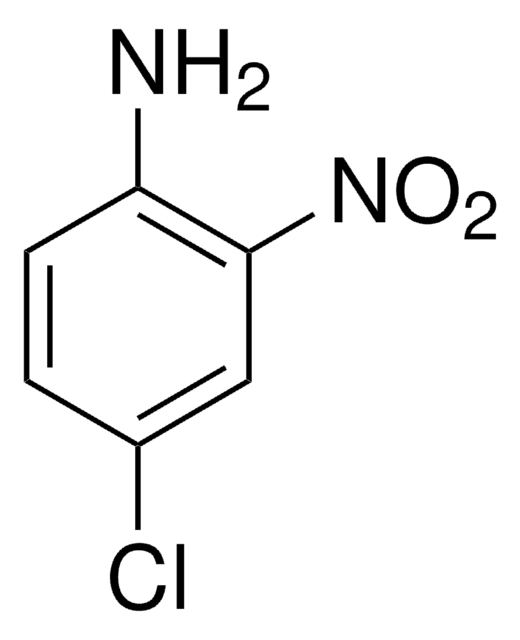
Linear Formula:
(O2N)2C6H3NH2
CAS Number:
Molecular Weight:
183.12
Beilstein:
982999
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
contains
≤15% water
mp
176-178 °C (lit.)
SMILES string
Nc1ccc(cc1[N+]([O-])=O)[N+]([O-])=O
InChI
1S/C6H5N3O4/c7-5-2-1-4(8(10)11)3-6(5)9(12)13/h1-3H,7H2
InChI key
LXQOQPGNCGEELI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Chronic 2 - STOT RE 2
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
435.2 °F - closed cup
Flash Point(C)
224 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
José Raul Herance et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 11(22), 6491-6502 (2005-08-12)
Zeolites are suitable microporous hosts for positively charged organic species, but it is believed that they cannot adsorb organic anions. Pure Meisenheimer complex, derived from reduction of 2,4-dinitroaniline with NaBH4, was adsorbed inside faujasite cavities. Evidence for the internal incorporation
C Bolognesi et al.
Bollettino della Societa italiana di biologia sperimentale, 56(23), 2480-2485 (1980-12-15)
The DNA damage induced by in vivo administration of: 2,4-dinitroaniline, ortho-toluidine and para-toluidine, was determined using alkaline filter elution of DNA. The target organs for ultimate carcinogens produced in mice appear to be the liver and the kidney. The damage
Xiaojia Huang et al.
Journal of chromatography. A, 1216(20), 4354-4360 (2009-04-03)
A simple and sensitive method for the determination of polar aromatic amines (PAAs) was developed using stir bar sorptive extraction (SBSE) coupling to high-performance liquid chromatography. A hydrophilic poly(vinylimidazole-divinylbenzene) (VIDB) monolithic material was prepared and acted as SBSE coating. The
O Zelenko et al.
Nucleic acids research, 22(14), 2731-2739 (1994-07-25)
The synthesis and enzymatic characterization of DUPAAA, a novel fluorogenic substrate for RNases of the pancreatic type is described. It consists of the dinucleotide uridylyl-3',5'-deoxyadenosine to which a fluorophore, o-aminobenzoic acid, and a quencher, 2,4-dinitroaniline, have been attached by means
T Azuma et al.
Biochemistry, 27(16), 6116-6120 (1988-08-09)
The interaction of M315 with 2,4-dinitrophenyl haptens was studied. 2,4-Dinitroaniline (DNP-NH2) showed maximum affinity to M315 at about pH 4. The pH dependence of the association constant of DNP-NH2 to M315 showed three transitions at pH 4.7, at pH 7.2
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service