All Photos(2)
About This Item
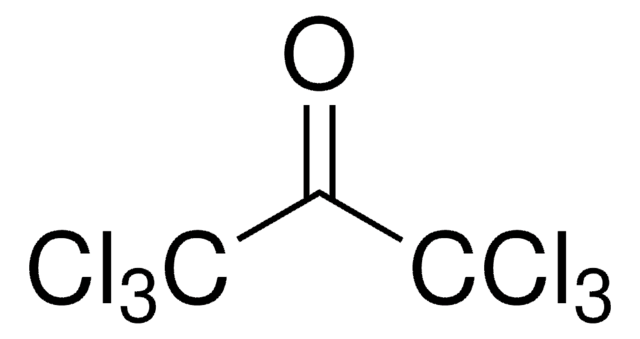
Linear Formula:
BrCCl3
CAS Number:
Molecular Weight:
198.27
Beilstein:
1732543
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
vapor density
6.85 (vs air)
vapor pressure
38.4 mmHg ( 25 °C)
Assay
99%
form
liquid
refractive index
n20/D 1.5065 (lit.)
bp
105 °C (lit.)
mp
−6 °C (lit.)
density
2.012 g/mL at 25 °C (lit.)
SMILES string
ClC(Cl)(Cl)Br
InChI
1S/CBrCl3/c2-1(3,4)5
InChI key
XNNQFQFUQLJSQT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Chain transfer agent for radical polymerization of methacrylates. Brominating reagent.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron, 50, 31-31 (1994)
Macromolecules, 27, 5863-5863 (1994)
C K Moon et al.
Drug and chemical toxicology, 15(1), 81-91 (1992-01-01)
Brazilin, the main constituent of Caesalpinia sappan, is an antioxidative substance that has catechol moiety in its chemical structure. Considering the antioxidant-activity of brazilin, it was expected to have protective effects on the toxicities of radical generating chemicals. The incubation
G D Castro et al.
Free radical research, 23(5), 431-442 (1995-11-01)
Free radicals generated by benzoyl peroxide-mediated catalytic decomposition of bromotrichloromethane (eg. trichloromethyl) were allowed to react under nitrogen or under air with uracil. Under nitrogen two reaction products were formed, one was identified as 5-chlorouracil and the other as a
Journal of the Chemical Society. Chemical Communications, 747-747 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service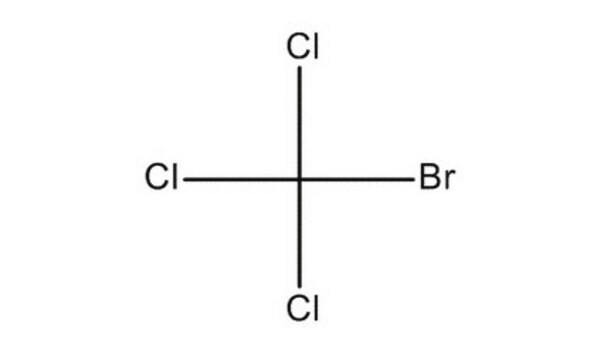


![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)