911178
PS-750-M
solution, 3 wt. % in H2O
Synonym(s):
FI-750-M solution
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Recommended Products
form
liquid
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
concentration
3 wt. % in H2O
refractive index
n/D 1.3373
density
0.9931 g/mL
greener alternative category
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced to increase catalytic efficiency and also acts as an environmentally benign and sustainable amphiphile. Click here for more information.
Application
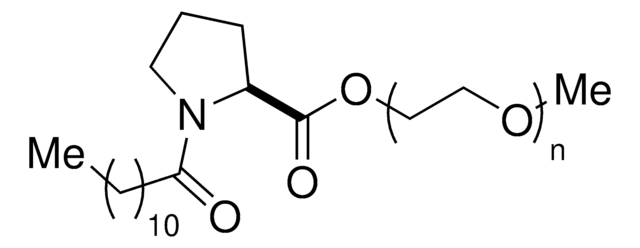
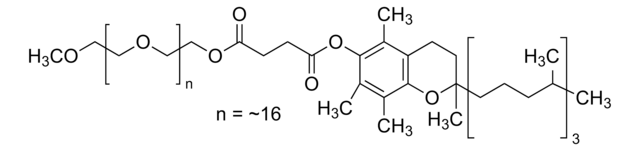
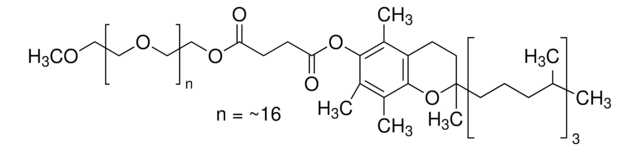
PS-750-M 3% solution in water is a custom surfactant developed in the Handa lab.
PS-750-M allows for a variety of reactions, including challenging cross-couplings and monofluorination of indoles, to be conducted in water.
PS-750-M allows for a variety of reactions, including challenging cross-couplings and monofluorination of indoles, to be conducted in water.
related product
Product No.
Description
Pricing
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Micelle-Enabled Palladium Catalysis for Convenient sp2-sp3 Coupling of Nitroalkanes with Aryl Bromides in Water Under Mild Conditions.
Brals J, et al.
ACS Catalysis, 7245-7250 (2017)
Micelle-Enabled Palladium Catalysis for Convenient sp2-sp3 Coupling of Nitroalkanes with Aryl Bromides in Water Under Mild Conditions.
Brals J, et al.
ACS Catalysis, 7(10), 7245-7250 (2017)
Lucie Finck et al.
The Journal of organic chemistry, 83(14), 7366-7372 (2018-02-10)
Using micelles of FI-750-M, visible light, photocatalysts, and inexpensive halogenating reagents, such as N-bromosuccinimide and N-chlorosuccinimde, selective oxyhalogenations of alkynes were achieved in water under very mild conditions. No halogenation at the aromatic rings was detected, and control experiments revealed
Shielding Effect of Micelle for Highly Effective and Selective Monofluorination of Indoles in Water.
Pranjal P Bora et al.
ChemSusChem, 12(13), 3037-3042 (2019-03-06)
Highly selective direct monofluorination of indoles and arenes was developed through an approach that allows site-specific solubility of substrate and fluorine source in the micelle. This approach was highly selective for a broad range of substrates with excellent functional group
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service