All Photos(2)
About This Item
Empirical Formula (Hill Notation):
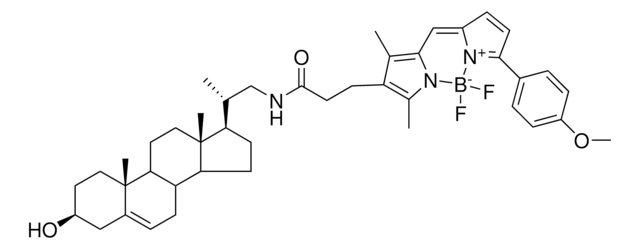
C45H39BF2N4O4
Molecular Weight:
748.62
UNSPSC Code:
12352111
NACRES:
NA.22
Recommended Products
Application
This compound is an Fmoc-labeled amino acid of tryptophan attached to a BODIPY dye through a spacer-free C-C linkage that can be used in solid phase peptide synthesis (SPPS). Research presented in Mendive-Tapia et al. tested the incorporation of the fluorescent amino acid into an anti-fungal peptide with two tryptophans by replacing either one or both of the residues. The three peptides showed enhanced fluorescence with increasing hydrophobicity of the environment. The peptides also maintained binding to Aspergillus fumigatus and could be detected by fluorescence imaging. That same publication showed that a cyclic version of the peptide containing a single fluorescent amino acid was more resistant to proteolysis than the linear peptide and still useful for imaging. The amino acid can be incorporated during standard conditions for SPPS and can also tolerate mildly acidic conditions of 1% trifluoroacetic acid allowing use of acid-labile solid supports. This amino acid should enable fluorescence detection of peptides for use in a variety of applications.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Spacer-free BODIPY fluorogens in antimicrobial peptides for direct imaging of fungal infection in human tissue.
Tapia ML, et al.
Nature Communications, 7, 10940-10940 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service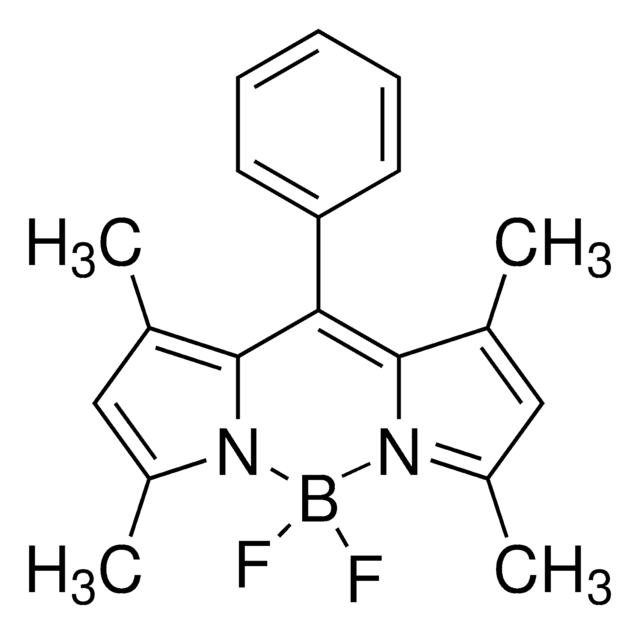
![Difluoro{2-[1-(3,5-dimethyl-2H-pyrrol-2-ylidene-N)ethyl]-3,5-dimethyl-1H-pyrrolato-N}boron 99% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/196/394/4c2c0eae-f749-44bf-a37b-84bf0226092e/640/4c2c0eae-f749-44bf-a37b-84bf0226092e.png)