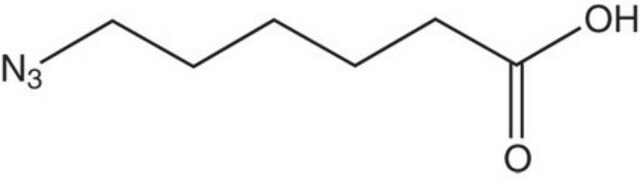
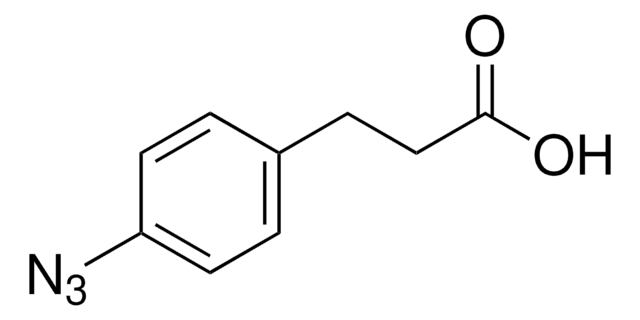
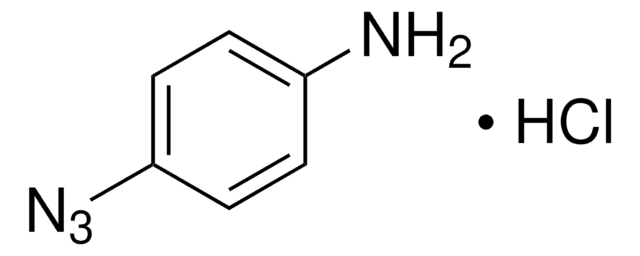
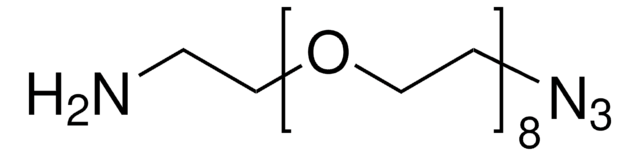
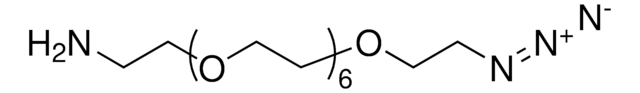
762016
3-Azido-1-propanamine
≥95%
Synonym(s):
3-Azidopropylamine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C3H8N4
CAS Number:
Molecular Weight:
100.12
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
form
liquid
reaction suitability
reaction type: click chemistry
refractive index
n20/D 1.471
density
1.020 g/mL at 25 °C
storage temp.
−20°C
SMILES string
NCCCN=[N+]=[N-]
InChI
1S/C3H8N4/c4-2-1-3-6-7-5/h1-4H2
InChI key
OYBOVXXFJYJYPC-UHFFFAOYSA-N
General description
3-Azido-1-propanamine can be used to functionalize:
- Bismethylolpropionic acid (bis-MPA) monomers with azide functional group to generate high-generation dendrimers.,
- Clickable zinc tetraphenylporphyrin scaffold with an azido group through click chemistry applicable in photodynamic therapy.
Application
Amine modified azide for click chemistry.
3-Azido-1-propanamine may be used in the synthesis of mannopyranoside dendrimers for studying multivalent carbohydrate-protein interactions.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
140.0 °F
Flash Point(C)
60 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of mono-, di-and triporphyrin building blocks by click chemistry for photodynamic therapy application
Gazzali AM, et al.
Tetrahedron, 73(5), 532-541 (2017)
Synthesis and solvodynamic diameter measurements of closely related mannodendrimers for the study of multivalent carbohydrate?protein interactions.
Chabre YM, et al.
Beilstein Journal of Organic Chemistry, 10, 1524-1524 (2014)
Two-dimensional ultrafast vibrational spectroscopy of azides in ionic liquids reveals solute-specific solvation
Dutta S, et al.
Physical Chemistry Chemical Physics, 17(40), 26575-26579 (2015)
Rapid Synthesis of Functionalized High-Generation Polyester Dendrimers via Strain-Promoted Alkyne-Azide Cycloaddition
McNelles SA and Adronov Al
Macromolecules, 50(20), 7993-8001 (2017)
Mariano Ortega-Muñoz et al.
Nanoscale, 11(16), 7850-7856 (2019-04-10)
Activated carbon nanodots functionalized with acid anhydride groups (AA-CNDs) are prepared by one-pot water-free green thermolysis of citric acid. As a proof of concept of their capabilities as appealing and versatile platforms for accessing engineering nanoconstructs, the as-prepared AA-CNDs have
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service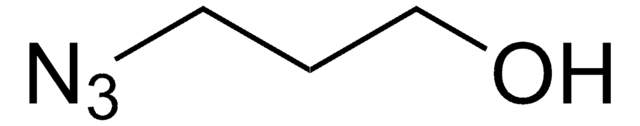


![2-[2-(2-Azidoethoxy)ethoxy]ethanol solution ~0.5 M in tert-butyl methyl ether](/deepweb/assets/sigmaaldrich/product/structures/374/007/eea7ca74-41e4-4aac-af71-c93c37ec0a5a/640/eea7ca74-41e4-4aac-af71-c93c37ec0a5a.png)