70444
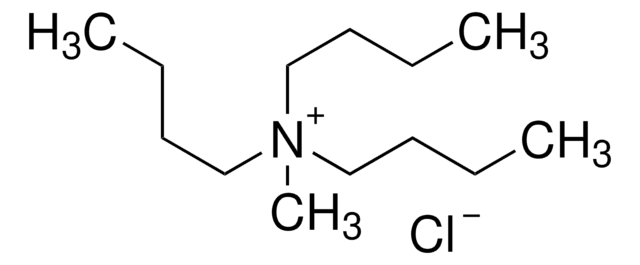
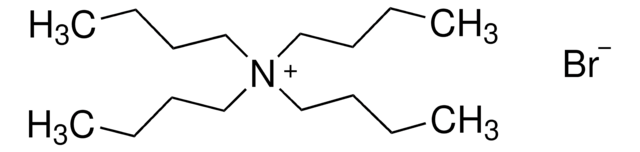
Tributylmethylammonium chloride
≥98.0% (T)
Synonym(s):
Methyltributylammonium chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3CH2CH2CH2)3N(Cl)CH3
CAS Number:
Molecular Weight:
235.84
Beilstein:
6300212
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (T)
form
crystals
SMILES string
[Cl-].CCCC[N+](C)(CCCC)CCCC
InChI
1S/C13H30N.ClH/c1-5-8-11-14(4,12-9-6-2)13-10-7-3;/h5-13H2,1-4H3;1H/q+1;/p-1
InChI key
IPILPUZVTYHGIL-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Tributylmethylammonium chloride is a quaternary ammonium salt commonly used as a catalyst in the synthesis of ɛ-caprolactone and 1-substituted tetrazoles.
Application
Tributylmethylammonium chloride can be used as a phase transfer catalyst in the synthesis of ɛ-caprolactone by Baeyer-Villiger oxidation of cyclohexanone in the presence of KHSO5 as an oxidizing agent.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Min-Koo Choi et al.
Journal of pharmaceutical sciences, 94(2), 317-326 (2004-12-01)
The in vivo canalicular excretion clearance of tributylmethyl ammonium (TBuMA), a P-glycoprotein (P-gp) substrate, was previously reported to be unaffected by the induction of an experimental hepatic injury (EHI) by CCl(4) despite the increased expression of P-gp in the EHI
J W Smit et al.
British journal of pharmacology, 123(3), 361-370 (1998-03-21)
1. In the present study it was tested whether known P-glycoprotein (P-gp) substrates/MDR reversal agents interact with small (type 1) and bulky (type 2) cationic drugs at the level of biliary excretion in the rat isolated perfused liver model (IPRL).
J E van Montfoort et al.
The Journal of pharmacology and experimental therapeutics, 298(1), 110-115 (2001-06-16)
Previous inhibition studies with taurocholate and cardiac glycosides suggested the presence of separate uptake systems for small "type I" (system1) and for bulky "type II" (system2) organic cations in rat hepatocytes. To identify the transport systems involved in type I
Soon-Sun Hong et al.
Archives of pharmacal research, 29(4), 323-327 (2006-05-10)
The objective of this study was to examine the pharmacokinetics of organic cations in intrahepatic cholestatic rats. A pretreatment with 17alpha-ethynylestradiol was used to induce intrahepatic cholestasis, and tributylmethylammonium (TBuMA) was used as a representative model organic cation. When [3H]TBuMA
Soon-Sun Hong et al.
Archives of pharmacal research, 29(4), 318-322 (2006-05-10)
Many quaternary ammonium salts are incompletely absorbed after their oral administration and may also be actively secreted into the intestine. However, the underlying mechanism(s) that control the transport of these cations across the intestinal epithelium is not well understood. In
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service