593656
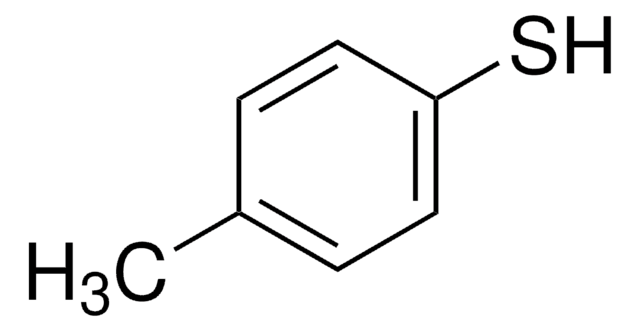
4-tert-Butylbenzenethiol
97%
Synonym(s):
4-tert-Butylthiophenol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3CC6H4SH
CAS Number:
Molecular Weight:
166.28
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.5480 (lit.)
bp
238 °C (lit.)
density
0.964 g/mL at 25 °C (lit.)
SMILES string
CC(C)(C)c1ccc(S)cc1
InChI
1S/C10H14S/c1-10(2,3)8-4-6-9(11)7-5-8/h4-7,11H,1-3H3
InChI key
GNXBFFHXJDZGEK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-tert-Butylbenzenethiol can be used to synthesize Pd–thiolate complex by coordinating with Pd2+.The reduction of this complex forms ultrasmall palladium nanoclusters.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
>230.0 °F - closed cup
Flash Point(C)
> 110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sakiat Hossain et al.
Nanoscale, 11(45), 22089-22098 (2019-11-14)
2-Phenylethanethiolate (PET) and 4-tert-butylbenzenethiolate (TBBT) are the most frequently used ligands in the study of thiolate (SR)-protected metal clusters. However, the effect of difference in the functional group between these ligands on the fundamental properties of the clusters has not
Masaaki Yusa et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 16(7), 1043-1048 (2017-05-10)
Under the irradiation of red light (690 nm), quinones were converted to hydroquinones by thiols in the presence of metallophthalocyanines. The reaction proceeded via the charge separation between the triplet state of phthalocyanine and the quinone. The product determining step
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service