All Photos(1)
About This Item
Empirical Formula (Hill Notation):
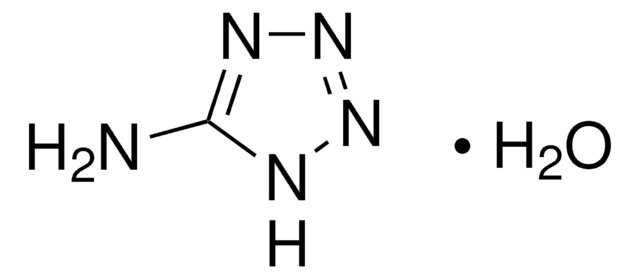
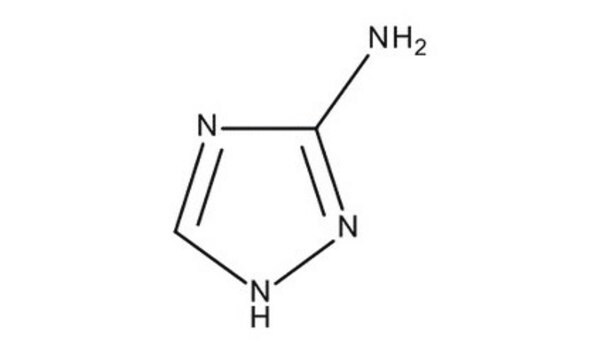
CH3N5
CAS Number:
Molecular Weight:
85.07
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
201-205 °C (lit.)
SMILES string
Nc1nnn[nH]1
InChI
1S/CH3N5/c2-1-3-5-6-4-1/h(H3,2,3,4,5,6)
InChI key
ULRPISSMEBPJLN-UHFFFAOYSA-N
General description
5-Aminotetrazole (5-AT) can react with different 2-ethoxymethylidene-3-oxo esters and their analogs to form biologically important azaheterocycles.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Desen. Expl. 4 - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
An efficient synthesis of tetrahydrotetrazolo [1, 5-a] quinazoline derivatives by a three-component reaction of 5-aminotetrazole, arylaldehydes, and dimedone.
Hassankhani A and Mosaddegh E.
Scientia Iranica. Transaction C, Chemistry, Chemical Engineering, 22(3), 942-942 (2015)
Selda Abyar et al.
Scientific reports, 9(1), 14686-14686 (2019-10-13)
Complexes based on heavy metals have great potential for the treatment of a wide variety of cancers but their use is often limited due to toxic side effects. Here we describe the synthesis of two new cadmium complexes using N(4)-phenyl-2-formylpyridine
S Thomas et al.
The journal of physical chemistry. A, 109(44), 9928-9934 (2006-07-15)
The surface-enhanced Raman scattering (SERS) studies of 5-amino tetrazole (5AT), a tetrazole derivative, in aqueous silver sol at pH approximately 9 and on deposited colloidal silver films were carried out and compared with the normal Raman spectrum of the molecule.
Jian-Guo Zhang et al.
Journal of molecular modeling, 15(1), 67-77 (2008-10-23)
The tautomerism and intramolecular hydrogen shifts of 5-amino-tetrazole in the gas phase were studied in the present work. The minimum energy path (MEP) information of 5-amino-tetrazole was obtained at the CCSD(T)/6-311G**//MP2/6-311G** level of theory. The six possible tautomers of 1H
W C de Bruijn et al.
The Histochemical journal, 16(1), 37-50 (1984-01-01)
Addition of heterocyclic nitrogen compounds to the classical osmium tetroxide postfixation medium, applied after glutaraldehyde fixation, results in enhanced membrane contrast in ultrathin sections of liver tissue. The addition of similar compounds to potassium osmate solutions, results in contrast differences
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service