488712
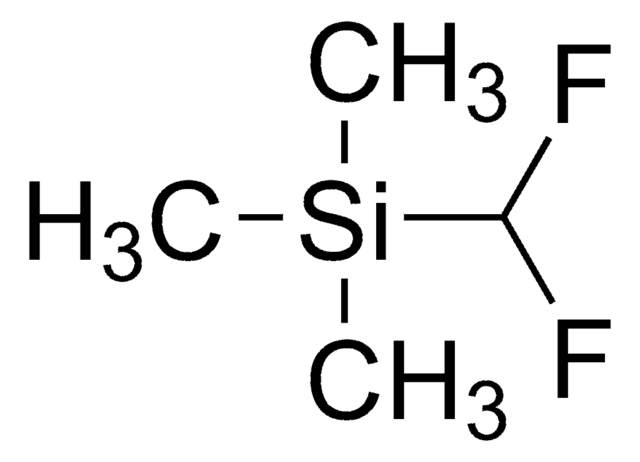
Trimethyl(trifluoromethyl)silane
99%
Synonym(s):
(Trifluoromethyl)trimethylsilane, Ruppert’s Reagent, TFMTMS
About This Item
Recommended Products
Quality Level
Assay
99%
reaction suitability
reaction type: C-C Bond Formation
bp
54-55 °C (lit.)
density
0.962 g/mL at 20 °C (lit.)
functional group
fluoro
storage temp.
2-8°C
SMILES string
C[Si](C)(C)C(F)(F)F
InChI
1S/C4H9F3Si/c1-8(2,3)4(5,6)7/h1-3H3
InChI key
MWKJTNBSKNUMFN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Conversion of N-(tert-butylsulfinyl)-imines to trifluoromethylated amines
- Conversion of trans-enones to trans-α-trifluoromethyl silyl ethers
- Trifluoromethylation of azomethine imines
- Conversion of H-phosphonates to CF3-phosphonates
- Nucleophilic addition of the trifluoromethyl group to aldehydes and ketones.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - Water-react 2
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service