488216
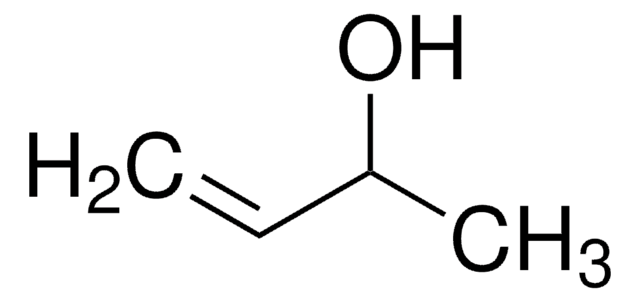
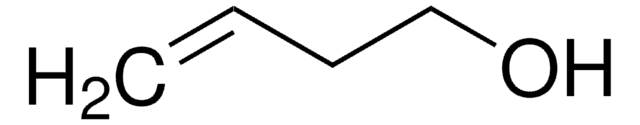
3,4-Dihydroxy-1-butene
≥99%
Synonym(s):
3-Butene-1,2-diol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH2=CHCH(OH)CH2OH
CAS Number:
Molecular Weight:
88.11
Beilstein:
1633578
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
bp
195 °C/733 mmHg (lit.)
density
1.047 g/mL at 25 °C (lit.)
SMILES string
OCC(O)C=C
InChI
1S/C4H8O2/c1-2-4(6)3-5/h2,4-6H,1,3H2
InChI key
ITMIAZBRRZANGB-UHFFFAOYSA-N
General description
3,4-Dihydroxy-1-butene, also known as 3-butene-1,2-diol (BDdiol), is a metabolite of 1,3-butadiene. It forms the precursor for synthesizing different chiral building blocks. BDdiol can undergo oxidation to form hydroxymethylvinyl ketone (HMVK). 1,2-epoxy-3-butene (EB) on hydrolysis in the presence of epoxide hydrolases (EH) forms BDdiol.
Application
3,4-Dihydroxy-1-butene can be used:
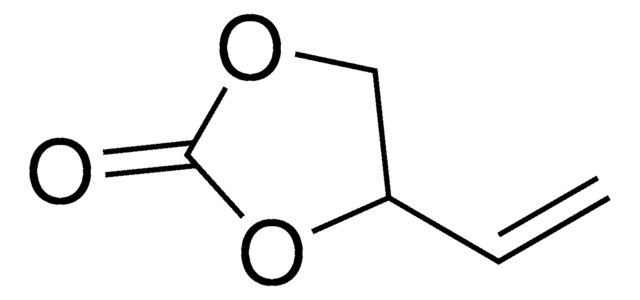
- As a reactant to synthesize cyclic organic carbonates by continuous flow procedure.
- To prepare substituted oxazolidinone ligands used to target medicinally relevant RNAs.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
3-Butene-1, 2-diol: An attractive precursor for the synthesis of enantiomerically pure organic compounds.
Rao AVR, et al.
Tetrahedron, 45(22), 7031-7040 (1989)
Versatile and scalable synthesis of cyclic organic carbonates under organocatalytic continuous flow conditions
Gerardy R, et al.
Catalysis Science & Technology, 9(24), 6841-6851 (2019)
R A Kemper et al.
Chemical research in toxicology, 9(7), 1127-1134 (1996-10-01)
3-Butene-1,2-diol (BDD) is a metabolite of the carcinogenic petrochemical 1,3-butadiene. BDD is produced by cytochrome P450-mediated oxidation of 1,3-butadiene to butadiene monoxide, followed by enzymatic hydrolysis by epoxide hydrolase. The metabolic disposition of BDD is unknown. The current work characterizes
Christopher L Sprague et al.
Toxicological sciences : an official journal of the Society of Toxicology, 80(1), 3-13 (2004-05-07)
3-Butene-1,2-diol (BDD) is a major metabolite of 1,3-butadiene (BD), but the role of BDD in BD toxicity and carcinogenicity remains unclear. In this study, the acute toxicity of BDD was investigated in male Sprague-Dawley rats and B6C3F1 mice. Of the
E Malvoisin et al.
Xenobiotica; the fate of foreign compounds in biological systems, 12(2), 137-144 (1982-02-01)
1. In rat liver microsomes, 1,3-butadiene was metabolized to butadiene monoxide, which was subsequently transformed into 3-butene-1,2-diol by microsomal epoxide hydrolase. 2. In the metabolism of butadiene oxide in microsomes, four metabolites were detected, namely two stereoisomers of DL-diepoxybutane, and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service