431974
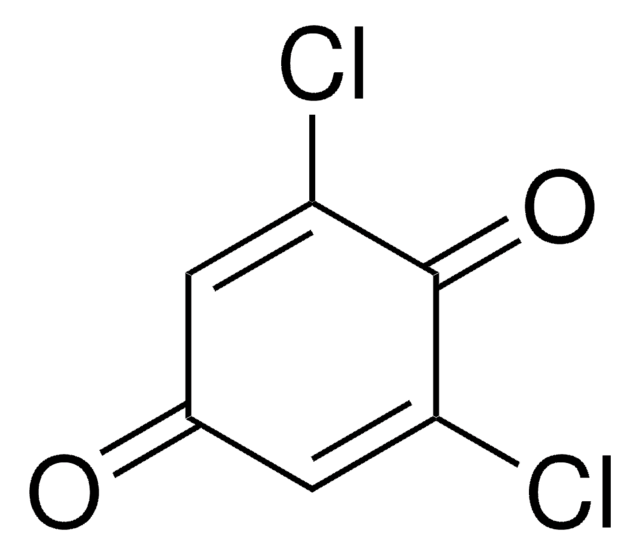
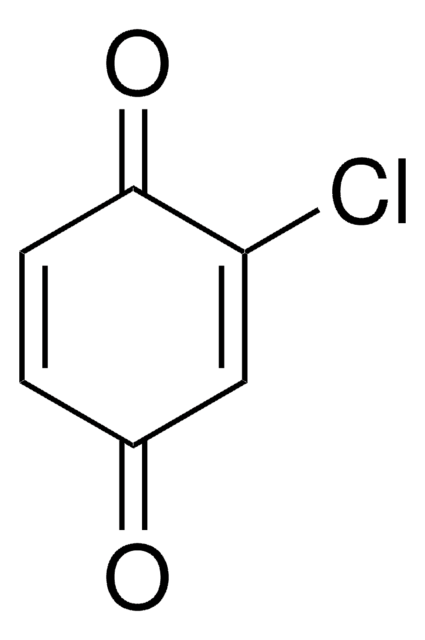
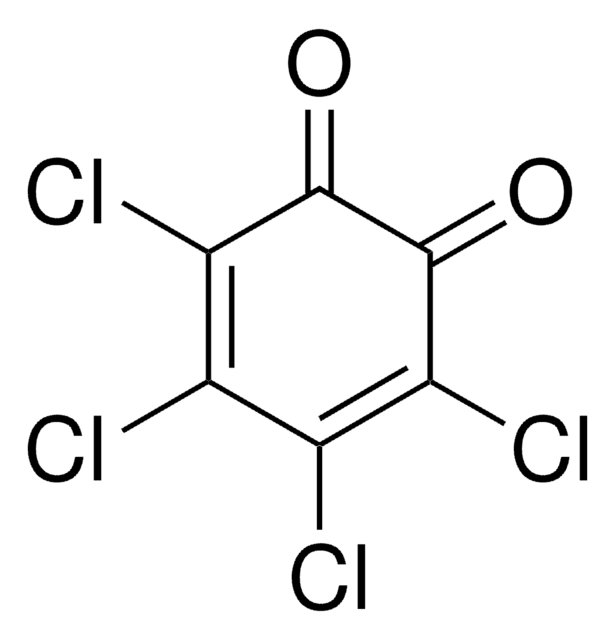
2,5-Dichloro-1,4-benzoquinone
98%
Synonym(s):
2,5-Dichloro-2,5-cyclohexadiene-1,4-dione, 2,5-Dichloro-p-benzoquinone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H2Cl2O2
CAS Number:
Molecular Weight:
176.98
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
160-163 °C (lit.)
functional group
chloro
ketone
SMILES string
ClC1=CC(=O)C(Cl)=CC1=O
InChI
1S/C6H2Cl2O2/c7-3-1-5(9)4(8)2-6(3)10/h1-2H
InChI key
LNXVNZRYYHFMEY-UHFFFAOYSA-N
General description
2,5-Dichloro-1,4-benzoquinone (DCBQ) is a halogenated quinone. DCBQs are carcinogenic intermediates.They have benn identified as chlorination disinfection byproducts in drinking water. DCBQ has been reported to increse the decomposition of a model ROOH tert-butylhydroperoxide, via formation of t-butoxyl radicals. The isomers of the DCBQ dimer have been investigated for the non-covalent interactions (NCIs) by quantum chemical calculations. Halogen bond present in 2,5-dichloro-1,4-benzoquinone have been investigated by experimental as well as theoretical charge density analysis. Its reaction with pyrrolidine has been investigated.
Application
2,5-Dichloro-1,4-benzoquinone may be used in the following processes:
- As a starting material in the synthesis of asterriquinone D.
- As a model to study the utility of a novel photoreactor with LED (light-emitting diode) light source and a fibre-optic CCD (charge-coupled device) spectrophotometer.
- 2,5-dichloro-3,6-bi(3-indolyl)-1,4-hydroquinone synthesis by palladium catalyzed reaction with indole.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
B Vijaya Pandiyan et al.
Physical chemistry chemical physics : PCCP, 16(37), 19928-19940 (2014-08-15)
The competition between non-covalent interactions (NCIs), such as C-H∙∙∙O, C-H∙∙∙Cl, C-Cl∙∙∙O, C-Cl∙∙∙Cl-C, C-O∙∙∙C, C-Cl∙∙∙C and C-O∙∙∙π, in the isomers of the 2,5-dichloro-1,4-benzoquinone (DCBQ) dimer were investigated by quantum chemical calculations to study the properties of the ground and excited states.
Halogen bonding in 2, 5-Dichloro-1, 4-benzoquinone: Insights from experimental and theoretical charge density analysis.
Hathwar VR, et al.
Crystal Growth & Design, 11(5), 1855-1862 (2011)
Chun-Hua Huang et al.
Chemical research in toxicology, 28(5), 831-837 (2015-03-20)
Halogenated quinones (XQ) are a class of carcinogenic intermediates and newly identified chlorination disinfection byproducts in drinking water. Organic hydroperoxides (ROOH) can be produced both by free radical reactions and enzymatic oxidation of polyunsaturated fatty acids. ROOH have been shown
Virág Kiss et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 16(4), 519-526 (2016-12-13)
Substituted 1,4-benzoquinone (QR) derivatives are photosensitive in aqueous solution and form hydroquinones (QR-H
Construction of a photochemical reactor combining a CCD spectrophotometer and a LED radiation source.
Gombar M, et al.
Photochemical & Photobiological Sciences : Official Journal of the European Photochemistry Association and the European Society for Photobiology, 11(10), 1592-1595 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service