283746
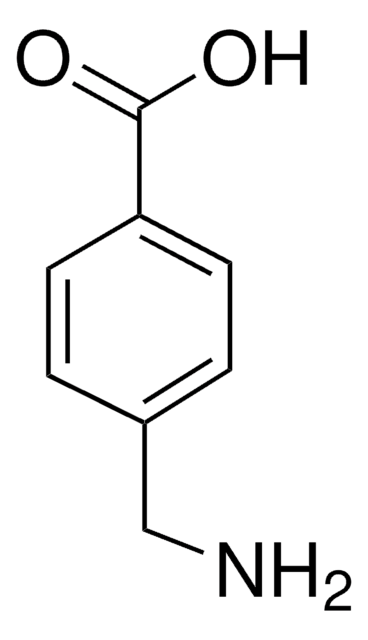
4-(Aminomethyl)benzoic acid
97%, for peptide synthesis
Synonym(s):
PAMBA
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Linear Formula:
H2NCH2C6H4CO2H
CAS Number:
Molecular Weight:
151.16
Beilstein:
1100606
EC Number:
MDL number:
UNSPSC Code:
12352106
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Product Name
4-(Aminomethyl)benzoic acid, 97%
Quality Level
Assay
97%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
mp
≥300 °C (lit.)
application(s)
peptide synthesis
SMILES string
NCc1ccc(cc1)C(O)=O
InChI
1S/C8H9NO2/c9-5-6-1-3-7(4-2-6)8(10)11/h1-4H,5,9H2,(H,10,11)
InChI key
QCTBMLYLENLHLA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-(Aminomethyl)benzoic acid (PAMBA) can be used in the synthesis of:
- Cobalt carboxy phosphonates.
- Apoptozole (Az), which has cellular potency to promote membrane trafficking of mutant cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel activity.
- Cyclopeptide composed of L-glutamic acid and 3-aminobenzoic acid that can be used as receptors for a variety of cations and anions.
- A bioactive peptide which has potent GPR54 (a G protein-coupled receptor) agonistic activity.
Other Notes
4-(Aminomethyl)benzoic acid is also an antihemorrhagic agent, which is used for the treatment of internal haemorrhage.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sandra Osburn et al.
Rapid communications in mass spectrometry : RCM, 21(21), 3409-3419 (2007-09-29)
Formation of [bn+17+cat]+ is a prominent collision-induced dissociation (CID) pathway for Li+- and Na+-cationized peptides. Dissociation of protonated and Ag+-cationized peptides instead favors formation of the rival bn+/[bn-1+cat]+ species. In this study the influence of a 4-aminomethylbenzoic acid (4AMBz) residue
E M Gorskaia et al.
Zhurnal mikrobiologii, epidemiologii, i immunobiologii, (1)(1), 87-90 (1995-01-01)
The possibility of the correction of intestinal microflora disorders and the functional activity of macrophages in dysbiosis, caused by the intragastric administration of ampiox, with the use of amben (PAMBA), an inhibitor of proteolytic enzymes, was studied. Quantitative and qualitative
Carlos F R A C Lima et al.
The journal of physical chemistry. A, 111(42), 10598-10603 (2007-10-05)
The standard (p0 = 0.1 MPa) molar enthalpies of combustion of six aminomethylbenzoic acids were measured at T = 298.15 K by static bomb calorimetry. With these values, the standard molar enthalpies of formation in the crystalline state were obtained.
M N Iakushenko et al.
Zhurnal mikrobiologii, epidemiologii, i immunobiologii, (1)(1), 77-79 (1998-04-09)
The examination of 49 newborn infants revealed that at the early neonatal period the character of the microbial colonization of the intestine depended on the kind of perinatal pathology: in lesions of the central nervous system and conjugation jaundice the
G A Chalyĭ et al.
Zhurnal mikrobiologii, epidemiologii, i immunobiologii, (1)(1), 62-65 (1993-01-01)
The influence of protease-inhibiting preparations on the development of humoral immune response in diseases involving the development of secondary immunodeficiency (experimentally induced acute pancreatitis and staphylococcal infection) has been studied. Five injections of contrycal and epsilon-aminocaproic acid (epsilon-ACA), starting from
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service