All Photos(2)
About This Item
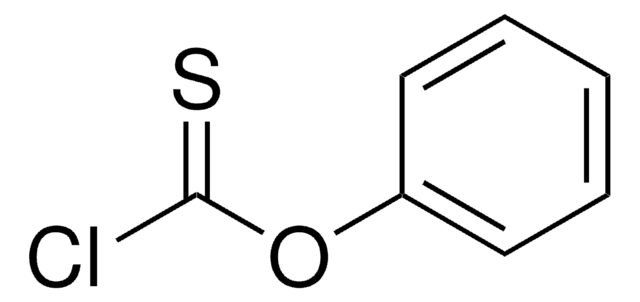
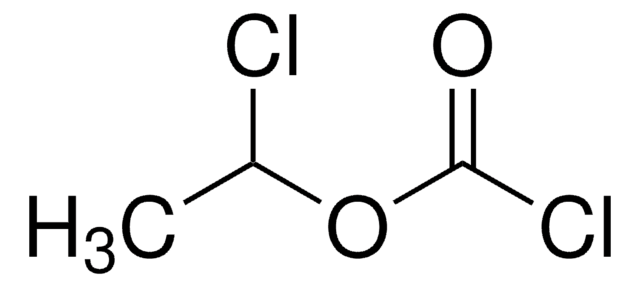
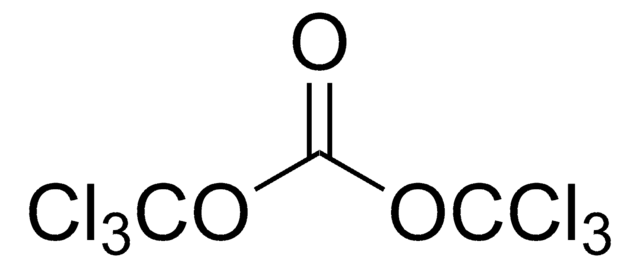
Linear Formula:
ClC(S)OC6H4CH3
CAS Number:
Molecular Weight:
186.66
Beilstein:
1448986
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0%
refractive index
n20/D 1.573 (lit.)
n20/D 1.573
bp
245 °C (lit.)
50 °C/0.2 mmHg (lit.)
density
1.225 g/mL at 25 °C (lit.)
functional group
chloro
storage temp.
2-8°C
SMILES string
Cc1ccc(OC(Cl)=S)cc1
InChI
1S/C8H7ClOS/c1-6-2-4-7(5-3-6)10-8(9)11/h2-5H,1H3
InChI key
UNCAXIZUVRKBMN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
O-(p-Tolyl) chlorothionoformate has been used in the synthesis of:
- α-L-2′-deoxythreofuranosyl nucleoside analogs
- alkenes from hindered alcohols
Other Notes
Synthesis of alkenes from hindered alcohols
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kiran S Toti et al.
European journal of medicinal chemistry, 46(9), 3704-3713 (2011-06-15)
The synthesis of a series of α-L-2'-deoxythreofuranosyl nucleosides featuring the nucleobases A, T, C and U is described in seven steps from 1,2-O-isopropyledene-α-L-threose, involving a Vorbrüggen coupling and a Barton-McCombie deoxygenation protocol as the key steps. All analogues, including a
H. Gerlach et al.
Journal of the Chemical Society. Chemical Communications, 1215-1215 (1972)
H. Gerlach et al.
Helvetica Chimica Acta, 55, 2277-2277 (1972)
A.P. Neary et al.
Journal of the Chemical Society. Chemical Communications, 1090-1090 (1989)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service