All Photos(1)
About This Item
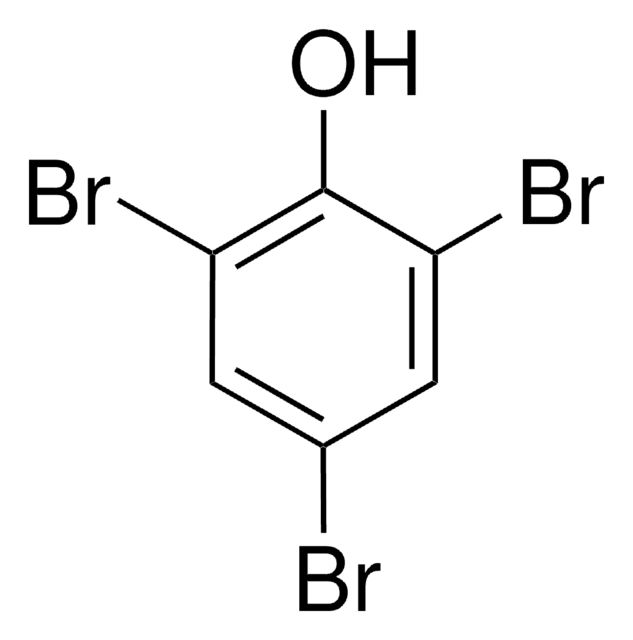
Linear Formula:
Br2C6H3OH
CAS Number:
Molecular Weight:
251.90
Beilstein:
1861291
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
bp
154 °C/11 mmHg (lit.)
mp
35-38 °C (lit.)
functional group
bromo
SMILES string
Oc1ccc(Br)cc1Br
InChI
1S/C6H4Br2O/c7-4-1-2-6(9)5(8)3-4/h1-3,9H
InChI key
FAXWFCTVSHEODL-UHFFFAOYSA-N
Gene Information
human ... ALOX12(239) , ALOX15(246)
Looking for similar products? Visit Product Comparison Guide
General description
The electrochemical hydrodehalogenation of 2,4-dibromophenol has been studied by electrochemical reduction in H-cells and solid polymer electrolyte cells using catalyzed cathodes. 2,4-Dibromophenol inhibits the microbial activity in marine sediments.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Inhibition of microbial activity in marine sediments by a bromophenol from a hemichordate.
King GM.
Nature, 323(6085), 257-259 (1986)
H Cheng et al.
Environmental science & technology, 38(2), 638-642 (2004-01-31)
A new technology for remediation of halogenated organics-oil systems, which can cause serious environmental problems, has been demonstrated using the electrochemical hydrodehalogenation of 2,4-dibromophenol (DBP) in paraffin oil in a solid polymer electrolyte reactor. The reactor has been evaluated in
G M King
Applied and environmental microbiology, 54(12), 3079-3085 (1988-12-01)
Halophenols such as 2,4-dibromophenol (DBP) occur naturally in some marine sediments, as a consequence of various animal and algal activities. In an earlier study, DBP was observed in the burrow microenvironment of the hemichordate Saccoglossus kowalewskii. At the concentrations found
Hui Liu et al.
Chemosphere, 84(4), 512-518 (2011-04-05)
Hydroxylated polybrominated diphenyl ethers (HO-PBDEs) have received significant attention due to their toxicities and universal presence in the environmental matrices. However, their origins are not fully understood. We explored the feasibility of the generation of HO-PBDEs through photochemical processes from
Alpha-glucosidase inhibitory activity of bromophenol purified from the red alga Polyopes lancifolia.
Keun Young Kim et al.
Journal of food science, 75(5), H145-H150 (2010-07-16)
A bromophenol, bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, was purified from the red alga Polyopes lancifolia. Its IC(50) values were 0.098 and 0.120 microM against Saccharomyces cerevisiae and Bacillus stearothermophilus alpha-glucosidases, respectively, and 1.00 and 1.20 mM against rat-intestinal sucrase and maltase. This bromophenol
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 258164-100G | 4061826061435 |
| 258164-500G | |
| 258164-5G | 4061826061459 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service