230669
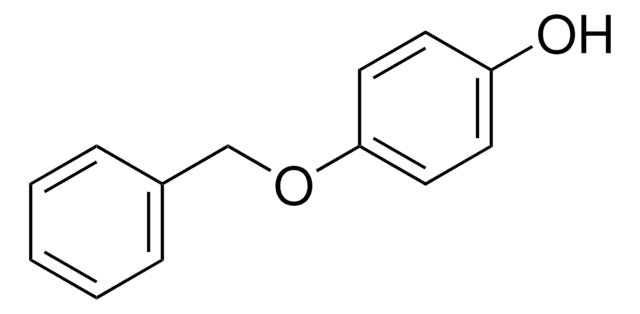
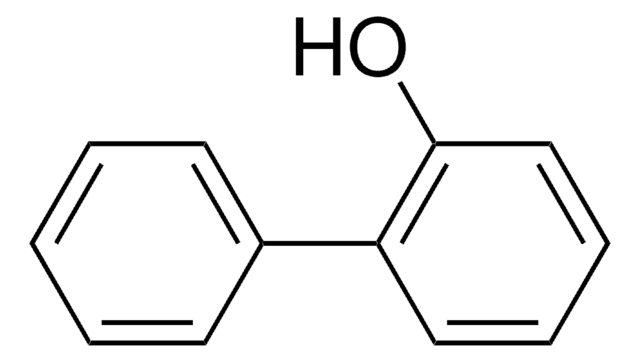
4-Phenoxyphenol
99%
Synonym(s):
Hydroquinone monophenyl ether
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5OC6H4OH
CAS Number:
Molecular Weight:
186.21
Beilstein:
2047182
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
80-84 °C (lit.)
functional group
phenoxy
SMILES string
Oc1ccc(Oc2ccccc2)cc1
InChI
1S/C12H10O2/c13-10-6-8-12(9-7-10)14-11-4-2-1-3-5-11/h1-9,13H
InChI key
ZSBDGXGICLIJGD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
338.0 °F - closed cup
Flash Point(C)
170 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Victoria B F Custodis et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(36), 8658-8668 (2017-04-08)
One of the key challenges in renewable chemical production is the conversion of lignin, especially by fast pyrolysis. The complexity of the lignin pyrolysis process has hindered the elucidation of the mechanism, inhibiting further industrial implementation. By combining pyrolysis of
Kyoungseon Min et al.
Biotechnology for biofuels, 10, 212-212 (2017-09-16)
In the biorefinery utilizing lignocellulosic biomasses, lignin decomposition to value-added phenolic derivatives is a key issue, and recently biocatalytic delignification is emerging owing to its superior selectivity, low energy consumption, and unparalleled sustainability. However, besides heme-containing peroxidases and laccases, information
Xiaolu Jiang et al.
Bioorganic & medicinal chemistry letters, 18(24), 6549-6552 (2008-10-28)
The synthesis and biological evaluation of a series of diphenyl ether derivatives were described. The compounds can either activate or inhibit the aminopeptidase activity of leukotriene A(4) hydrolase, while at the same time do not influence the hydrolase activity. Further
Cynthia D Selassie et al.
Journal of medicinal chemistry, 48(23), 7234-7242 (2005-11-11)
In this comprehensive study on the caspase-mediated apoptosis-inducing effect of 51 substituted phenols in a murine leukemia cell line (L1210), we determined the concentrations needed to induce caspase activity by 50% (I50) and utilized these data to develop the following
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service