163872
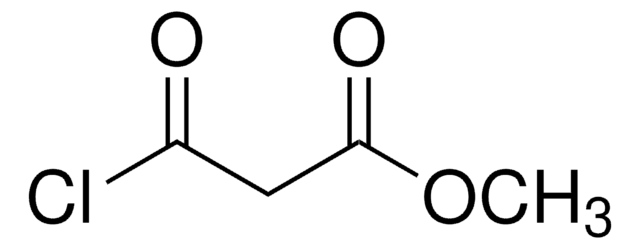
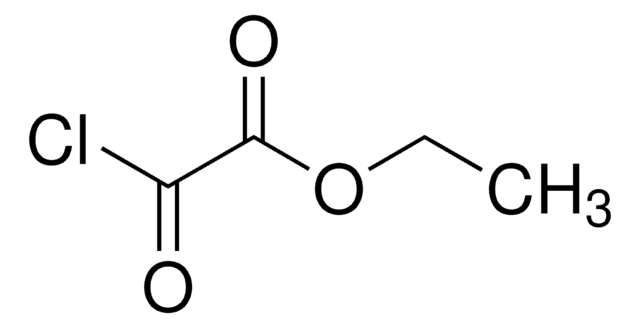
Ethyl malonyl chloride
technical grade
Synonym(s):
Ethyl (chloroformyl)acetate, Ethyl 3-chloro-3-oxopropionate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
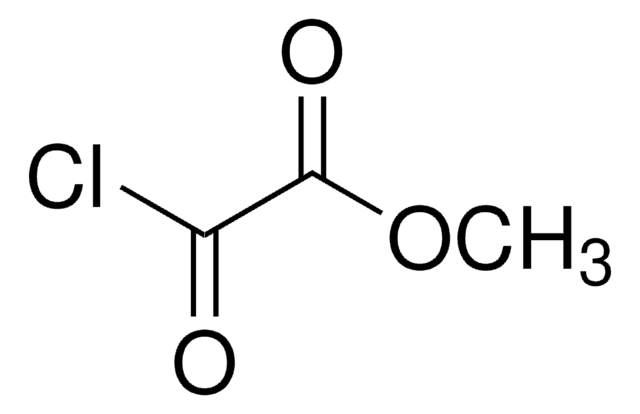
CH3CH2OCOCH2COCl
CAS Number:
Molecular Weight:
150.56
Beilstein:
636215
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
refractive index
n20/D 1.429 (lit.)
bp
79-80 °C/25 mmHg (lit.)
density
1.176 g/mL at 25 °C (lit.)
functional group
acyl chloride
ester
storage temp.
2-8°C
SMILES string
CCOC(=O)CC(Cl)=O
InChI
1S/C5H7ClO3/c1-2-9-5(8)3-4(6)7/h2-3H2,1H3
InChI key
KWFADUNOPOSMIJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Ethyl malonyl chloride is a versatile acylating agent for propargyl alcohols, hydrazines and amines.
Application
Ethyl malonyl chloride was used in the synthesis of:
- liquid-crystalline methanofullerodendrimers
- 3,5-disubstituted 1,2,4-oxadiazole derivatives, which are potential peptidomimetic building blocks
- 3-pyrrolin-2-ones via amidation with propargylamines and subsequent base catalyzed 5-exo-dig cyclization
Versatile acylating agent for propargyl alcohols, hydrazines, and amines.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
147.2 °F - closed cup
Flash Point(C)
64 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
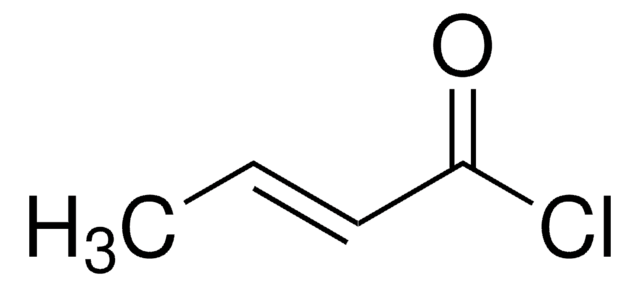
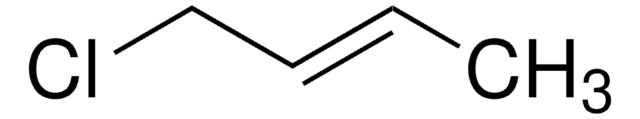
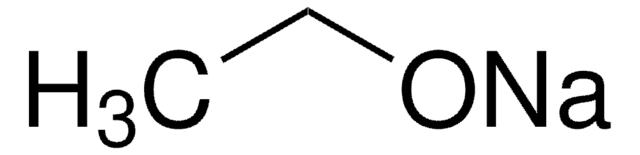
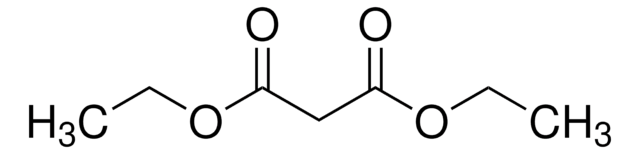
Customers Also Viewed
Sudarat Yenjai et al.
Journal of photochemistry and photobiology. B, Biology, 186, 23-30 (2018-07-11)
Rational design of photoreagents with systematic modifications of their structures can provide valuable information for a better understanding of the protein photocleavage mechanism by these reagents. Variation of the length of the linker connecting the photoactive moiety with the protein
Liquid-crystalline methanofullerodendrimers which display columnar mesomorphism.
Maringa N, et al.
Journal of Materials Chemistry, 18(13), 1524-1534 (2008)
N A Meanwell et al.
Journal of medicinal chemistry, 36(24), 3871-3883 (1993-11-26)
The 4,5-diphenyloxazole derivatives 2-4 were previously identified as nonprostanoid prostacyclin (PGI2) mimetics. A series of derivatives of 2-4 bearing substitutents at the carbon atom alpha to the oxazole ring were synthesized and evaluated as inhibitors of ADP-induced aggregation of human
Synlett, 65-65 (1993)
Synthesis of 3, 5-disubstituted 1, 2, 4-oxadiazoles as peptidomimetic building blocks.
Jakopin Z, et al.
Tetrahedron Letters, 48(8), 1465-1468 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service