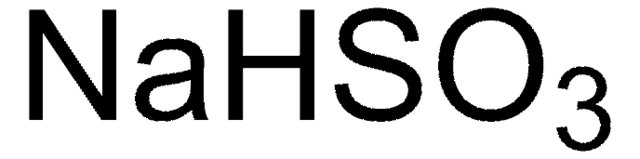
108790
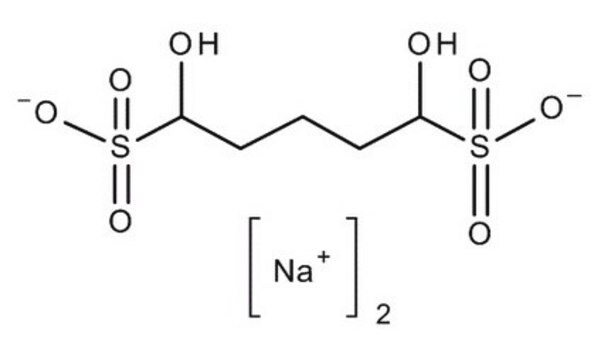
Glutaraldehyde sodium bisulfite addition compound
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Recommended Products
Quality Level
mp
>300 °C (lit.)
solubility
1 M NaOH: soluble 50 mg/mL, clear (colourless to dark yellow-orange)
1 M NaOH: soluble 50 mg/mL
SMILES string
OS(C(O)CCCC(O)S(O)(=O)=O)(=O)=O.[Na+].[Na+]
InChI
1S/C5H12O8S2.2Na/c6-4(14(8,9)10)2-1-3-5(7)15(11,12)13;;/h4-7H,1-3H2,(H,8,9,10)(H,11,12,13);;/q;2*+1/p-2
InChI key
YGZZDQOCTFVBFC-UHFFFAOYSA-L
Application
Glutaraldehyde may be used as a cross-linking reagent.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H Fan et al.
International journal of pharmaceutics, 213(1-2), 103-116 (2001-02-13)
Doxorubicin is one of the most potent anti-tumor agents used generally in the treatment of bone cancer. Like other cancer chemotharepeutics, it produces undesirable side effects such as cardiotoxicity, which is especially severe when administrated via the conventional intravenous route.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service