Cinchona Alkaloids
Asymmetric phase-transfer catalysis (PTC) has been recognized as a “green” alternative to many homogeneous synthetic organic transformations, and has found widespread application. Synthetically modified cinchona alkaloids are typical chiral organocatalysts used in asymmetric PTC. Several generations of O-alkyl N-arylmethyl derivatives were developed, which finally led to highly enantioselective alkylation reactions of glycine imines to generate a range of α-amino acid derivatives (Table 1).
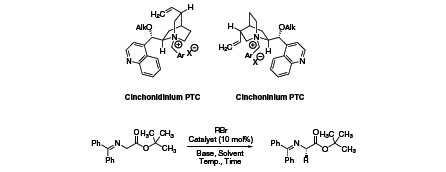
Figure 1. Alkylation reactions of glycine imines
In an attempt to further improve catalyst enantioselectivities, Jew and Park linked two cinchona alkaloid moieties via spacer units. With such a dimeric cinchona alkaloid (06542), enantioselectivity for the above mentioned glycine imine alkylation was optimized to 97–99% ee.1,2,3
Nucleophilic catalysts have had a wide-ranging role in the development of new synthetic methods. In particular, the cinchona alkaloids catalyze many useful processes with high enantioselectivities. Cinchona alkaloids can be used as bases to deprotonate substrates with relatively acidic protons forming a contact ion pair between the resulting anion and protonated amine. This interaction leads to a chiral environment around the anion and permits enantioselective reactions with electrophiles.
Important in many of these processes is the ability to control the formation of quaternary asymmetric centers with high enantiomeric excesses. Using the (DHQD)2AQN (456713) catalyst it is possible to affect the α-functionalization of ketones by the addition of TMSCN to the corresponding cyanohydrin in excellent yield and enantiomeric excess (Scheme 1).4
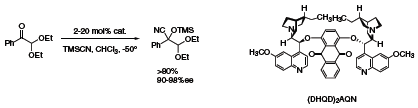
Scheme 1
The metal-free, allylic amination reaction provides a useful extension to the conventional palladium catalyzed π-allylic methodology. Amination with diimides at the remote γ-position can be carried out using (DHQ)2PYR (418978) (Scheme 2) to form a diverse range of highly functionalized amine compounds.5
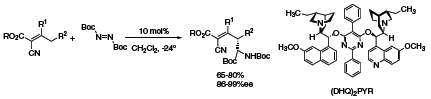
Scheme 2
Finally, Jørgensen and co-workers have developed the first catalytic enantioselective conjugate addition to alkynones using (DHQ)2PHAL (392723).6 For both aromatic and aliphatic alkynones the addition of β-diketones proceeds in high yields and enantioselectivity giving a mixture of (E)- and (Z)-enones (Scheme 3).
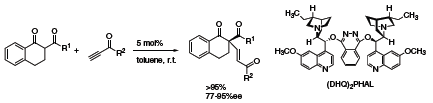
Scheme 3
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?