SAB4200695
Anti-VSV Glycoprotein antibody, Mouse monoclonal
clone P5D4, purified from hybridoma cell culture
Synonym(s):
VSV, VSV glycoprotein, Vesicular Stomatitis Virus glycoprotein
About This Item
IF
IP
immunocytochemistry: suitable
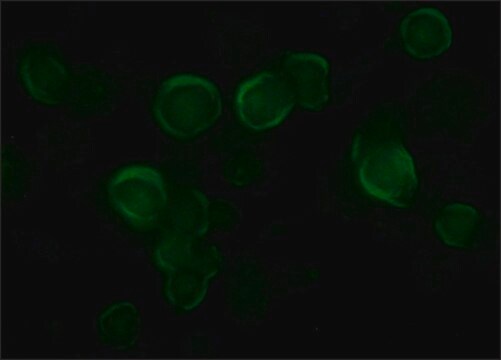
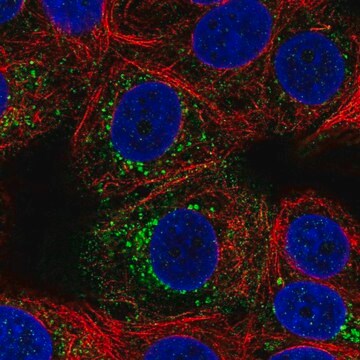
immunofluorescence: 5-10 μg/mL using COS7 cells over-expressing Vinculin with VSV-G tagged fusion protein.
immunoprecipitation (IP): suitable
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
P5D4, monoclonal
form
buffered aqueous solution
species reactivity
virus (Vesicular stomatitis virus (VSV))
packaging
antibody small pack of 25 μL
concentration
~1 mg/mL
technique(s)
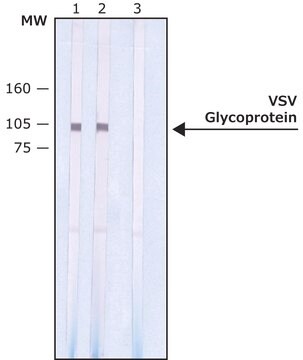
immunoblotting: 0.5-1 μg/mL using whole extract of human HEK-293T cells over-expressing Vinculin with VSV-G tagged fusion protein.
immunocytochemistry: suitable
immunofluorescence: 5-10 μg/mL using COS7 cells over-expressing Vinculin with VSV-G tagged fusion protein.
immunoprecipitation (IP): suitable
isotype
IgG1
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
General description
Application
Biochem/physiol Actions
Physical form
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
12 - Non Combustible Liquids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service