P7634
3-Phosphoglyceric Phosphokinase from baker′s yeast (S. cerevisiae)
ammonium sulfate suspension, ≥500 units/mg protein
Synonym(s):
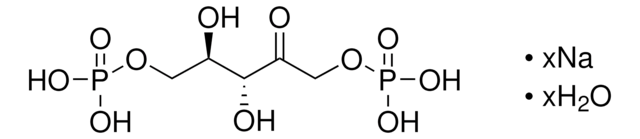
PGK, Phosphoglycerate kinase, 3-Phosphoglycerate kinase, ATP:3-Phospho-D-glycerate 1-phosphotransferase
About This Item
Recommended Products
biological source
bakers yeast
Quality Level
form
ammonium sulfate suspension
specific activity
≥500 units/mg protein
storage condition
(Tightly closed)
concentration
1.0-10.0 mg/mL
foreign activity
Glyceraldehyde-3-phosphate dehydrogenase ≤0.1%
shipped in
wet ice
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
General description
Phosphoglycerate kinase (PGK), a glycolytic enzyme, is isolated from a broad variety of organisms. This typical hinge-bending monomeric enzyme is well-conserved among the three domains of life. The enzyme is made up of a single folded polypeptide chain that divides into two nearly identical domains, each linked by two -helices (-helices 7 and 14) and separated by a deep cleft. This arrangement gives the enzyme its distinctive bilobed structure.
Application
- to study low molecular weight GTP-binding proteins and mechanisms of inhibition of glyceraldehyde-3-phosphate dehydrogenase
- in the coupled assay to measure the backward activity of purified rabbit skeletal muscle nicotinamide adenine dinucleotide (NAD+)-dependent glyceraldehyde3-phosphate dehydrogenase (GAPDH)
- in the assay of glyceraldehyde-3-phosphate dehydrogenase
Biochem/physiol Actions
Unit Definition
Physical form
Analysis Note
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Instructions for working with enzymes supplied as ammonium sulfate suspensions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service