All Photos(1)
About This Item
Empirical Formula (Hill Notation):
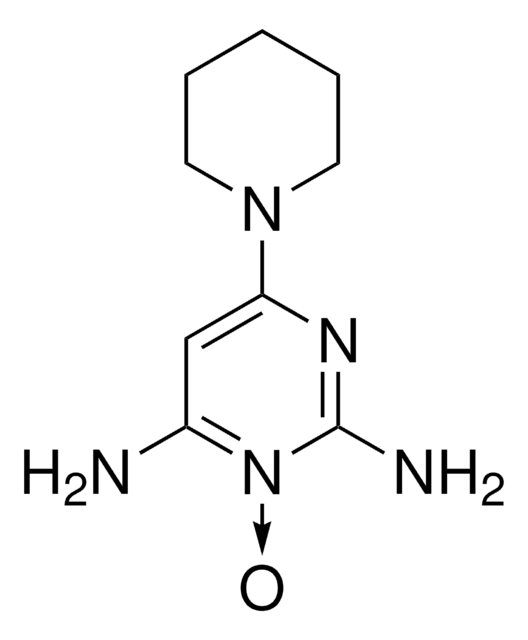
C9H15N5O4S
CAS Number:
Molecular Weight:
289.31
MDL number:
UNSPSC Code:
12352107
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
form
powder
Quality Level
originator
Johnson & Johnson
storage temp.
−20°C
SMILES string
Nc1cc(nc(N)[n+]1OS([O-])(=O)=O)N2CCCCC2
InChI
1S/C9H15N5O4S/c10-7-6-8(13-4-2-1-3-5-13)12-9(11)14(7)18-19(15,16)17/h6H,1-5H2,(H4,10,11,12,15,16,17)
InChI key
OEOLOEUAGSPDLT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Minoxidil sulfate (MXS) has been used as a drug agent to study its effects on alopecia in corticotropin-releasing factor over-expressing (CRF-OE) mice. It has also been used as a positive control in an assay for the culturing of rat vibrissa follicles.
Biochem/physiol Actions
Minoxidil sulfate (MXS) is an endogenous derivative of minoxidil. It possesses greater aqueous solubility and is a potent vasodilator. MXS has the potential to treat androgenic alopecia or male baldness.
Features and Benefits
This compound was developed by Johnson & Johnson. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
Other Notes
Active metabolite of minoxidil.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C E Ohrnberger et al.
The Journal of pharmacology and experimental therapeutics, 267(1), 25-30 (1993-10-01)
Glyburide, a sulfonylurea, and U-37883A, a guanidine (4-Morpholinecarboximidine-N-1-Adamantyl-N' cyclohexylhydrochloride), have been previously characterized as antagonists of the vascular ATP-sensitive K+ channels (KATP). In this report, the in vitro interaction between these two chemically distinct KATP antagonists was investigated using isolated
K Bray et al.
Naunyn-Schmiedeberg's archives of pharmacology, 345(2), 244-250 (1992-02-01)
The effects of the K+ channel blockers tedisamil and glibenclamide on cromakalim- and minoxidil sulphate-induced 42K+ and 86Rb+ efflux and vasorelaxation in rat aorta, were investigated. In aortic strips preloaded with 42K+ or 86Rb+, cromakalim (1 mumol/l) induced increases in
K S Atwal
Journal of cardiovascular pharmacology, 24 Suppl 4, S12-S17 (1994-01-01)
KATP openers are recognized as having a therapeutic potential for the treatment of various cardiovascular and noncardiovascular diseases. However, the first-generation agents open KATP in a variety of tissues that limit their potential clinical utility. This review describes our studies
K M Bray et al.
The Journal of biological chemistry, 267(17), 11689-11692 (1992-06-15)
The K+ channel openers, including cromakalim, pinacidil, minoxidil sulfate, diazoxide, and nicorandil, form a chemically heterogeneous group of compounds, which relax smooth muscle by opening plasmalemmal K+ channels. At present it is not known whether these drugs elicit their effects
K D Meisheri et al.
The Journal of pharmacology and experimental therapeutics, 266(2), 655-665 (1993-08-01)
This study describes the in vitro and in vivo characteristics of a guanidine 4-morpholinecarboximidine-N-1-adamantyl-N'-cyclohexyl-hydroc hloride (U-37883A), as an antagonist of vascular ATP-sensitive K+ channels (KATP). In isolated rabbit mesenteric artery, the antagonistic effects of U-37883A (0.5-5 microM) were studied against
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service