D9904
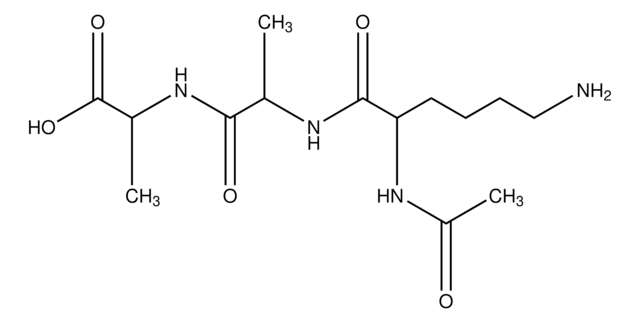
Nα,Nε-Diacetyl-Lys-D-Ala-D-Ala
carboxypeptidase substrate
Synonym(s):
(Ac)2-L-Lys-D-Ala-D-Ala
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H28N4O6
CAS Number:
Molecular Weight:
372.42
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
composition
Peptide content, ≥85%
solubility
water: 50 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
OC([C@@H](C)NC([C@@H](C)NC([C@@H](NC(C)=O)CCCCNC(C)=O)=O)=O)=O
InChI
1S/C16H28N4O6/c1-9(14(23)19-10(2)16(25)26)18-15(24)13(20-12(4)22)7-5-6-8-17-11(3)21/h9-10,13H,5-8H2,1-4H3,(H,17,21)(H,18,24)(H,19,23)(H,20,22)(H,25,26)
InChI key
VIHGYLJIMMKSBR-UHFFFAOYSA-N
Substrates
Substrate for penicillin-sensitive D-alanine carboxypeptidase.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Malabarba et al.
Journal of medicinal chemistry, 35(22), 4054-4060 (1992-10-30)
Basic carboxamides of teicoplanin A2 (CTA) and its aglycon (TD) are prepared by condensation of the 63-carboxyl function of these antibiotics with linear or branched polyamines. The antimicrobial activities of some of the resulting compounds were better than those of
Casey C McComas et al.
Journal of the American Chemical Society, 125(31), 9314-9315 (2003-08-02)
The binding affinity of 4, which incorporates a methylene (CH2) in place of the key linking amide of Ac2-l-Lys-d-Ala-d-Ala, for vancomycin was compared with that of Ac2-l-Lys-d-Ala-d-Ala (3) and Ac2-l-Lys-d-Ala-d-Lac (5). The vancomycin affinity for 4 was approximately 10-fold less
Cleidiane G Zampronio et al.
Analytical chemistry, 76(17), 5172-5179 (2004-09-18)
Electrospray ionization (ESI) is extensively used in the analysis of biological compounds; yet some fundamental properties of this technique are not completely understood. It is widely recognized that care should be exercised when noncovalent complexes are being studied by ESI
T R Herrin et al.
Journal of medicinal chemistry, 28(9), 1371-1375 (1985-09-01)
A series of ristocetin analogues with modifications (OH, C=O, C=NOH, NCOCH3) at the C-1' amino group was synthesized and found to possess antibacterial activity against gram-positive bacteria and to bind to Ac2-Lys-D-Ala-D-Ala, a model for the antibiotic's site of action.
M Nguyen-Distèche et al.
The Biochemical journal, 207(1), 109-115 (1982-10-01)
The membrane-bound, 26 000-Mr penicillin-binding protein of Streptomyces K15 has been isolated in the form of an effective, penicillin-sensitive D-alanyl-D-alanine-cleaving peptidase exhibiting high transpeptidase activity (greater than 95%) and very low carboxy-peptidase activity (less than 5%). The penicillin-binding protein/transpeptidase can
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service