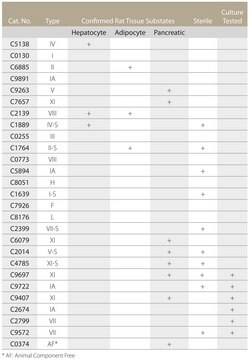
C8176
Collagenase from Clostridium histolyticum
Sigma Blend Type L, ≤1.0 FALGPA units/mg solid
Synonym(s):
Clostridiopeptidase A
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Recommended Products
Quality Level
form
powder
caseinase activity
≥40 units/mg solid
specific activity
≤1.0 FALGPA units/mg solid
mol wt
68-130 kDa
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Collagenase from Clostridium histolyticum, or Clostridiopeptidase A, has been used in a study to assess the synthesis and in vitro evaluation of macromolecular antitumour derivatives based on phenylenediamine mustard. Clostridiopeptidase A has also been used in a study to investigate the enzymatic debridement of burn wounds with collagenase.
Biochem/physiol Actions
Collagenase is activated by four gram atom calcium per mole enzyme. It is inhibited by ethylene glycol-bis(beta-aminoethyl ether) - N, N, N′,N′-tetraacetic acid, beta-mercaptoethanol, glutathione, thioglycolic acid and 8-hydroxyquinoline.
Effective release of cells from tissue requires the action of collagenase enzymes and the neutral protease. Collagenase is activated by four gram atom calcium (Ca2+) per mole enzyme. The culture filtrate is thought to contain at least 7 different proteases ranging in molecular weight from 68-130 kDa. The pH optimum is 6.3-8.8. The enzyme is typically used to digest the connective components in tissue samples to liberate individual cells. Ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA)4; β-mercaptoethanol; glutathione, reduced; thioglycolic acid, sodium; and 2,2′-dipyridyl; 8-hydroxyquinoline are known to inhibit the enzyme activity.
Unit Definition
One collagen digestion unit (CDU) liberates peptides from collagen from bovine achilles tendon equivalent in ninhydrin color to 1.0 μmole of leucine in 5 hours at pH 7.4 at 37 °C in the presence of calcium ions. One FALGPA hydrolysis unit hydrolyzes 1.0 μmole of furylacryloyl-Leu-Gly-Pro-Ala per min at 25°C. One Neutral Protease unit hydrolyzes casein to produce color equivalent to 1.0 μmole of tyrosine per 5 hr at pH 7.5 at 37°C. One Clostripain Unit hydrolyzes 1.0 μmole of BAEE per min at pH 7.6 at 25°C in the presence of DTT.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Coşkun Ozcan et al.
Burns : journal of the International Society for Burn Injuries, 28(8), 791-794 (2002-12-05)
Seventy-eight pediatric burn patients treated by enzymatic debridement with collagenase clostridiopeptidase A (CCA), were compared to 41 patients those burn wounds were excised surgically. Patients whose burn wounds were initially assessed as partial-thickness at admission were enrolled in the study.
N I Solov'eva et al.
Bioorganicheskaia khimiia, 20(3), 303-309 (1994-03-01)
Interactions of collagenases I and II (clostridiopeptidases) from Clostridium histolyticum with hexapeptide substrates in which some L-proline residues are replaced by their D-analogues, as well as with the tripeptide chloromethyl ketone Z-Gly-Pro-Gly-CH2Cl were studied. A role of stereochemistry of the
Ruth M Williams et al.
STAR protocols, 2(2), 100414-100414 (2021-04-20)
In order to process samples by fluorescence-activated cell sorting (FACS), it is essential to obtain a single-cell suspension of dissociated cells. Numerous protocols and commercial reagents are available; however, each requires optimization for specific tissue types. Here, we describe an
Dag K Skovseth et al.
Methods in molecular biology (Clifton, N.J.), 360, 253-268 (2006-12-19)
The future ability to manipulate the growth of new blood vessels (angiogenesis) holds great promise for treating ischemic disease and cancer. Several models of human in vivo angiogenesis have been described, but they seem to depend on transgenic support and
Katleen De Winne et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 24(2-3), 159-168 (2005-01-22)
Poly-[N-(2-hydroxyethyl)-L-glutamine] (PHEG) and poly(ethylene glycol) (PEG)-grafted PHEG conjugates of N,N-di(2-chloroethyl)-4-phenylenediamine mustard (PDM) were synthetised. A collagenase-sensitive oligopeptide spacer was selected to link the cytotoxic agent PDM onto the polymeric carrier. First, the oligopeptide-drug conjugate, L-pro-L-leu-gly-L-pro-gly-PDM, was prepared. In a second
Protocols
This procedure may be used for Collagenase products.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service