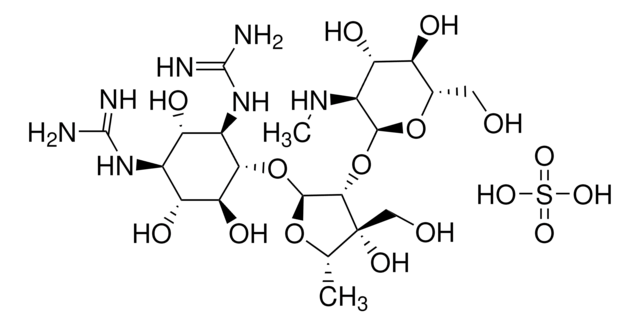
C4142
Capreomycin sulfate from Streptomyces capreolus
antibacterial peptide
About This Item
Recommended Products
Quality Level
form
powder
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
mycobacteria
Mode of action
DNA synthesis | interferes
storage temp.
−20°C
SMILES string
OS(O)(=O)=O.NCCC[C@H](N)C(=O)NC[C@H]1NC(=O)[C@H](CO)NC(=O)[C@H](N)CNC(=O)[C@@H](NC(=O)\C(NC1=O)=C\NC(N)=O)[C@H]2CCNC(=N)N2
InChI
1S/C24H42N14O8.H2O4S/c25-4-1-2-10(26)17(40)32-7-13-19(42)34-14(8-33-24(29)46)20(43)38-16(12-3-5-30-23(28)37-12)22(45)31-6-11(27)18(41)36-15(9-39)21(44)35-13;1-5(2,3)4/h8,10-13,15-16,39H,1-7,9,25-27H2,(H,31,45)(H,32,40)(H,34,42)(H,35,44)(H,36,41)(H,38,43)(H3,28,30,37)(H3,29,33,46);(H2,1,2,3,4)/b14-8-;/t10-,11+,12?,13+,15-,16-;/m0./s1
InChI key
LFFNIXQXRKNZCE-UBBQZPMLSA-N
Related Categories
Application
Biochem/physiol Actions
Preparation Note
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service