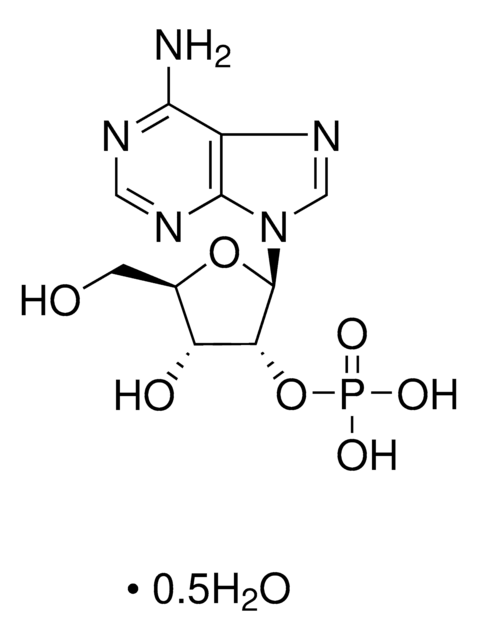
A1271
Adenosine 5′-monophosphate–Agarose
lyophilized powder
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
MDL number:
UNSPSC Code:
41106500
eCl@ss:
32160414
PubChem Substance ID:
NACRES:
NA.56
Recommended Products
biological source
plant (Sea weed)
form
lyophilized powder
extent of labeling
1-5 μmol per mL
matrix
cross-linked 4% beaded agarose
matrix activation
cyanogen bromide
matrix attachment
C-8
matrix spacer
9 atoms
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Adenosine 5′-monophosphate Agarose (5′-AMP agarose) has been used in affinity chromatography to isolate β and gamma glutamate decarboxylase, which is important for controlling gamma-aminobutyric acid (GABA) synthesis in brain.
Physical form
Lyophilized powder stabilized with lactose
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
F James et al.
The Journal of biological chemistry, 270(38), 22344-22350 (1995-09-22)
The plant enzyme S-adenosylmethionine:methionine S-methyltransferase (EC 2.1.1.12, MMT) catalyzes the synthesis of S-methylmethionine. MMT was purified 620-fold to apparent homogeneity from leaves of Wollastonia biflora. The four-step purification included fractionation with polyethylene glycol, affinity chromatography on adenosine-agarose, anion exchange chromatography
C D Murphy et al.
Applied and environmental microbiology, 67(10), 4919-4921 (2001-09-26)
Streptomyces cattleya is unusual in that it produces fluoroacetate and 4-fluorothreonine as secondary metabolites. We now report the isolation of an NAD(+)-dependent fluoroacetaldehyde dehydrogenase from S. cattleya that mediates the oxidation of fluoroacetaldehyde to fluoroacetate. This is the first enzyme
S J Wu et al.
Journal of neurochemistry, 42(6), 1607-1612 (1984-06-01)
The interactions of two forms of porcine brain glutamate decarboxylase (beta-GAD and gamma-GAD) with the effector ATP were studied by affinity chromatography. A third form, alpha-GAD, was only slightly retarded by the affinity matrix and was eluted in the buffer
M Kato et al.
Plant physiology, 120(2), 579-586 (1999-06-11)
Caffeine synthase (CS), the S-adenosylmethionine-dependent N-methyltransferase involved in the last two steps of caffeine biosynthesis, was extracted from young tea (Camellia sinensis) leaves; the CS was purified 520-fold to apparent homogeneity and a final specific activity of 5.7 nkat mg-1
D L Martin et al.
Journal of neurochemistry, 55(2), 524-532 (1990-08-01)
A major regulatory feature of brain glutamate decarboxylase (GAD) is a cyclic reaction that controls the relative amounts of holoenzyme and apoenzyme [active and inactive GAD with and without bound pyridoxal 5'-phosphate (pyridoxal-P, the cofactor), respectively]. Previous studies have indicated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service