PHR3656
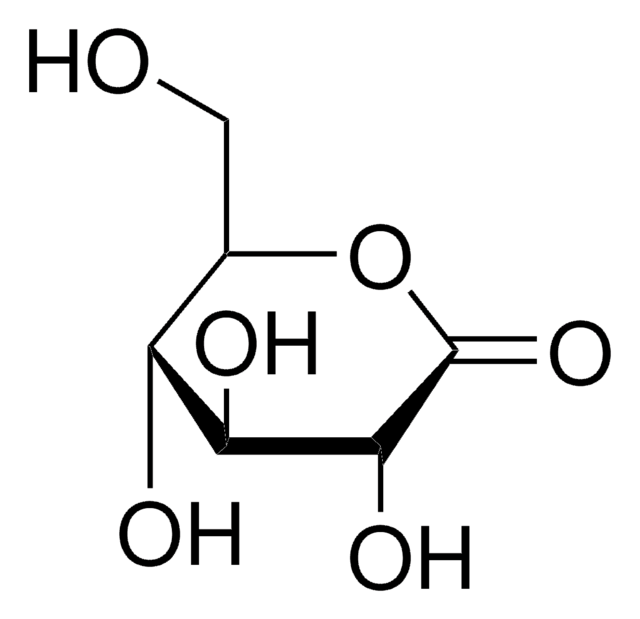
Gluconolactone
certified reference material, pharmaceutical secondary standard
Synonym(s):
1,5-Gluconolactone, D-(+)-Glucono-δ-lactone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H10O6
CAS Number:
Molecular Weight:
178.14
MDL number:
UNSPSC Code:
41116107
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
packaging
pkg of 200MG
storage temp.
2-30°C
InChI
1S/C6H10O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-5,7-10H,1H2
InChI key
PHOQVHQSTUBQQK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Application
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Analysis Note
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
Other Notes
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service