K3255
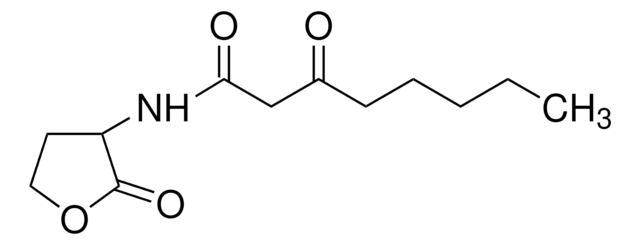
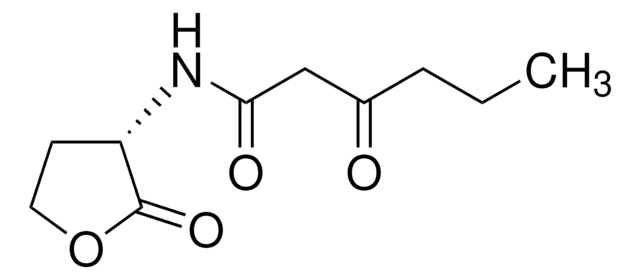
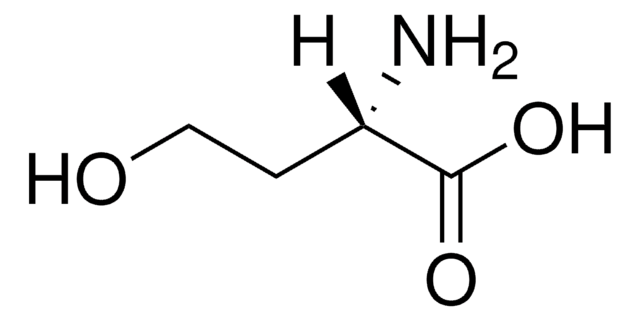
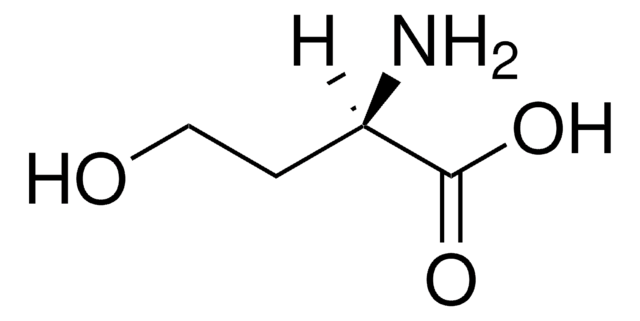
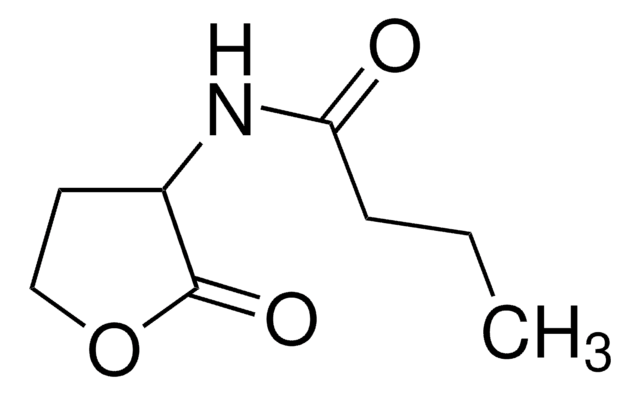
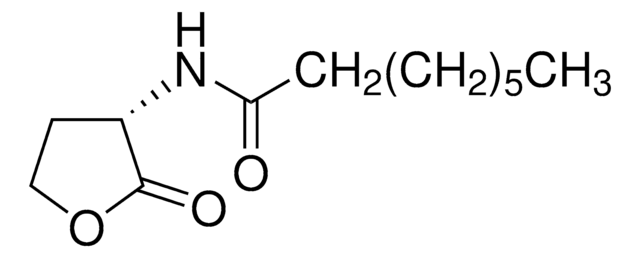
N-(β-Ketocaproyl)-DL-homoserine lactone
analytical standard
Synonym(s):
N-(3-Oxohexanoyl)-DL-homoserine lactone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H15NO4
CAS Number:
Molecular Weight:
213.23
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
suitability
suitable for manufacturing use
application(s)
forensics and toxicology
pharmaceutical (small molecule)
veterinary
format
neat
storage temp.
−20°C
SMILES string
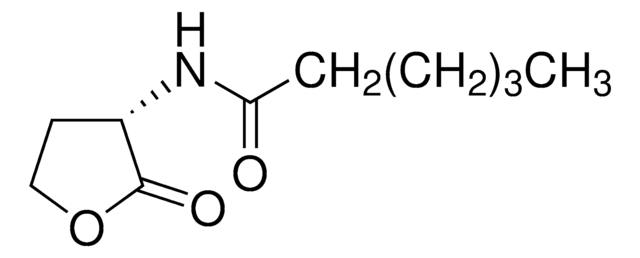
CCCC(=O)CC(=O)NC1CCOC1=O
InChI
1S/C10H15NO4/c1-2-3-7(12)6-9(13)11-8-4-5-15-10(8)14/h8H,2-6H2,1H3,(H,11,13)
InChI key
YRYOXRMDHALAFL-UHFFFAOYSA-N
Application
N-(β-Ketocaproyl)-DL-homoserine lactone has been used as a reference standard in identifying the quorum sensing signal molecules released by the in wood samples affected by Brenneria salicis bacteria bacteria, using ultra-high performance liquid chromatography coupled with an ion-trap mass spectrometer.
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Guoping Jin et al.
Biochemical and biophysical research communications, 417(3), 991-995 (2011-12-31)
Many Gram-negative bacteria use N-acyl-homoserine lactones (AHLs) as quorum sensing (QS) signaling molecules to coordinate their group behavior. Recently, it was shown that plants can perceive and respond to these bacterial AHLs. However, little is known about the molecular mechanism
Elena S Antonova et al.
FEMS microbiology letters, 322(1), 68-76 (2011-06-11)
Vibrio cholerae, the causative agent of cholera and a natural inhabitant of aquatic environments, regulates numerous behaviors using a quorum-sensing (QS) system conserved among many members of the marine genus Vibrio. The Vibrio QS response is mediated by two extracellular
Jessica R Gooding et al.
Biochemistry, 49(27), 5621-5623 (2010-06-10)
Extracellular autoinducer concentrations in cultures of Vibrio harveyi and Escherichia coli were monitored by liquid chromatography-tandem mass spectrometry to test whether a quantitative definition of quorum sensing could help decipher the information content of these signals. Although V. harveyi was
Di Lv et al.
Research in microbiology, 163(2), 119-124 (2011-12-08)
The phyllosphere is inhabited by large populations of epiphytic bacteria that are able to modulate their phenotypes and behavior by quorum sensing (QS). However, the impact of acyl-homoserine lactones (AHLs) involved in QS on the ecology of bacteria in their
Susanna Zucca et al.
BMC bioinformatics, 13 Suppl 4, S11-S11 (2012-05-02)
The bottom-up programming of living organisms to implement novel user-defined biological capabilities is one of the main goals of synthetic biology. Currently, a predominant problem connected with the construction of even simple synthetic biological systems is the unpredictability of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service