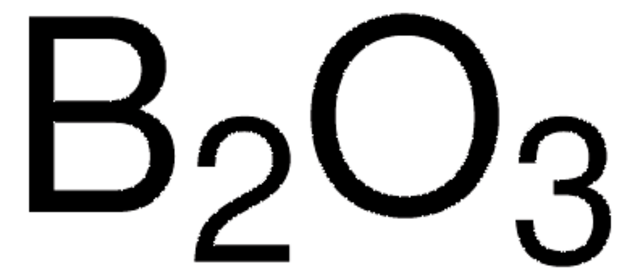339075
Boric anhydride
99.98% trace metals basis
Synonym(s):
Boron trioxide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
B2O3
CAS Number:
Molecular Weight:
69.62
EC Number:
MDL number:
UNSPSC Code:
12352300
eCl@ss:
38120103
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
grade
for inorganic trace analysis
Quality Level
vapor density
>1 (vs air)
Assay
99.98% trace metals basis
form
powder
mp
450 °C (lit.)
density
2.46 g/mL at 25 °C (lit.)
SMILES string
O=BOB=O
InChI
1S/B2O3/c3-1-5-2-4
InChI key
JKWMSGQKBLHBQQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Boric anhydride in sulfuric acid may be used as a scrubber to remove the hydrogen fluoride gas formed as a byproduct during the selective reduction of ketones to hydrocarbons using triethylsilane and boron trifluoride. In combination with hydroxyapatite, it may be used to catalyze the vapor-phase Beckmann rearrangement process to form nylon-6.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vapor-phase Beckmann rearrangement of cyclohexanone oxime over boria-hydroxyapatite catalyst
Izumi Y, et al.
Chemistry Letters (Jpn), 12(10), 1649-1652 (1983)
Reduction of ketones to hydrocarbons with triethylsilane: m-nitroethylbenzene
Fry JL, et al.
Organic Syntheses, 60, 108-108 (1981)
Jin-Su Nam et al.
Journal of hazardous materials, 172(2-3), 1013-1020 (2009-08-25)
Recently, it was found by the authors of this study that glasses of a special composition have an ability to remove some hazardous ions from waste solutions. In the present study, a SiO(2)-B(2)O(3)-CaO-Na(2)O glass system has been chosen to remove
Ivan Hung et al.
Journal of magnetic resonance (San Diego, Calif. : 1997), 197(2), 229-236 (2009-02-10)
Using a two-dimensional multiple-quantum (MQ) double rotation (DOR) experiment the contributions of the chemical shift and quadrupolar interaction to isotropic resonance shifts can be completely separated. Spectra were acquired using a three-pulse triple-quantum z-filtered pulse sequence and subsequently sheared along
G Krishna Kumari et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 101, 140-147 (2012-10-27)
Divalent Mn2+ ions containing 20ZnO+xLi2O+(30-x)K2O+50B2O3(5≤x≤25) mol% glasses are prepared by using melt quench technique and are characterized by several spectroscopic techniques. Various physical parameters are evaluated from the measured values of density and refractive index for the observation of mixed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service