1.03164
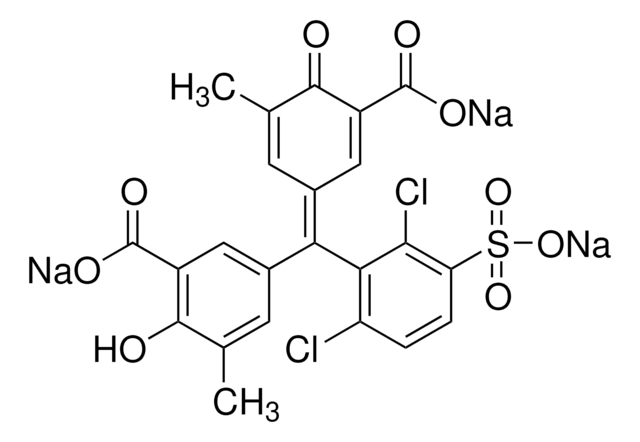
Eriochrome cyanine R (C.I. 43820)
for analysis (reagent for aluminium)
Synonym(s):
Eriochrome cyanine R (C.I. 43820)
About This Item
Recommended Products
Quality Level
potency
>2000 mg/kg LD50, oral (Rat)
loss
≤10% loss on drying, 110°C
pH
9.1 (24 °C, 10 g/L in H2O)
solubility
420 g/L
bulk density
590 kg/m3
λmax
434-440 nm (buffer pH 7.0)
storage temp.
2-30°C
InChI
1S/C23H18O9S.Na/c1-11-7-13(9-15(19(11)24)21(26)27)23(14-8-12(2)20(25)16(10-14)22(28)29)17-5-3-4-6-18(17)33(30,31)32-23;/h3-10,24-25H,1-2H3,(H,26,27)(H,28,29);/q;+1
InChI key
HJEGHSWGWSAZBS-UHFFFAOYSA-N
Related Categories
Application
- Determination of Food Oxalates Using Silica-Titania Xerogel Modified with Eriochrome Cyanine R.: This research explores a novel method to determine oxalate concentrations in food using Eriochrome Cyanine R, highlighting the dye′s potential in enhancing analytical sensitivity and specificity (Morosanova et al., 2018).
- Development of a Dispersive Liquid-Liquid Microextraction Method Combined with UV-Visible Spectrophotometry for Determination of Trace Aluminum(III) in Water, Wastewater, Food, Biological, and Pharmaceutical Samples.: This study demonstrates a new method utilizing Eriochrome Cyanine R for tracing aluminum levels across various samples, showcasing its utility in environmental and health-related chemical analyses (Birgani et al., 2017).
- Direct, sensitive determination of trace amounts of dissolved ferric iron in natural water by light absorption ratio variation spectrometry.: Though not directly mentioning Eriochrome Cyanine R, this article focuses on related spectrometric techniques that can be adapted for dyes like Eriochrome Cyanine R to detect iron in natural water sources (Gao et al., 2005).
- Determination of vanadium by solid-phase spectrophotometry after its preconcentration as an Eriochrome Cyanine R complex on a dextran-type exchanger.: This research introduces a method for vanadium determination using Eriochrome Cyanine R, underlining the dye′s role in the preconcentration and quantification of vanadium in complex matrices (Boudra et al., 1995).
Analysis Note
Absorption maximum λmax. (buffer pH 7.0): 434 - 440 nm
Spec. Absorptivity A 1%/1cm (λmax; 0.02 g/l; buffer pH 7.0; calc. on dried substance): 130 - 230
Loss on drying (110 °C): ≤ 10 %
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service