I9760
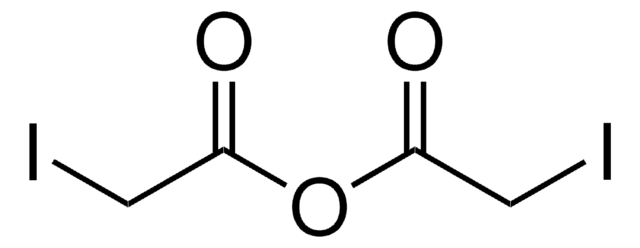
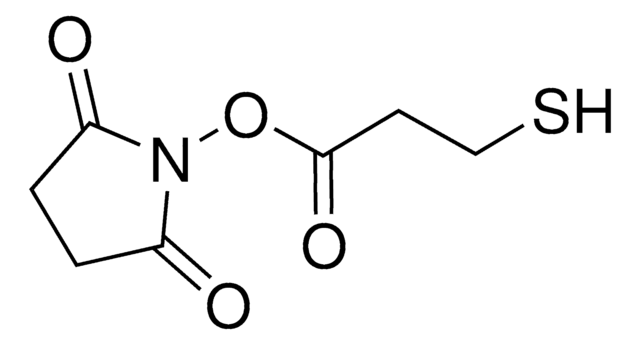
Iodoacetic acid N-hydroxysuccinimide ester
for peptide synthesis
Synonym(s):
N-Hydroxysuccinimide iodoacetate, N-Iodoacetoxysuccinimide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H6INO4
CAS Number:
Molecular Weight:
283.02
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Product Name
Iodoacetic acid N-hydroxysuccinimide ester, powder
form
powder
Quality Level
reaction suitability
reagent type: cross-linking reagent
solubility
DMF: 50 mg/mL
application(s)
peptide synthesis
functional group
NHS ester
storage temp.
−20°C
SMILES string
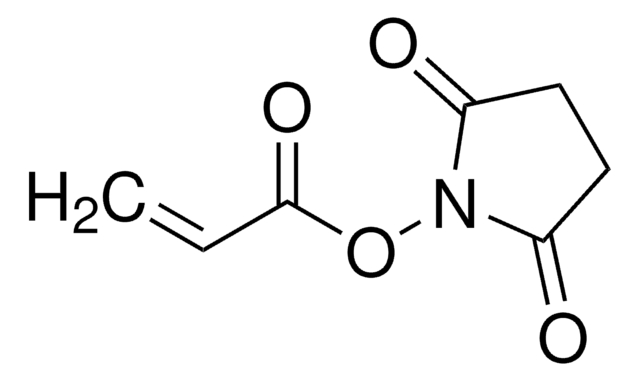
O=C(N1OC(CI)=O)CCC1=O
InChI
1S/C6H6INO4/c7-3-6(11)12-8-4(9)1-2-5(8)10/h1-3H2
InChI key
VRDGQQTWSGDXCU-UHFFFAOYSA-N
Application
A heterobifunctional cross-linking reagent with amine and sulfhydryl reactivity. Typically, coupled initially to molecules containing primary amines by amide bonds buffered at pH 7.5 (6.5-8.5) Second coupling specific for molecules containing free sulfydryl by thioether linkage buffered at pH 7.5 (7.0-8.0). Useful for preparation of enzyme immunoconjugates and hapten carrier molecule conjugates. Provides a 2-atom linker.
Caution
The iodoacetyl group is light sensitive.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kevin Y Lin et al.
ACS nano, 7(10), 9001-9009 (2013-09-11)
Thrombin is a serine protease and regulator of hemostasis that plays a critical role in the formation of obstructive blood clots, or thrombosis, that is a life-threatening condition associated with numerous diseases such as atherosclerosis and stroke. To detect thrombi
G Houen et al.
Journal of immunological methods, 181(2), 187-200 (1995-04-26)
Two methods for the preactivation of proteins and conjugation of peptides to proteins under mild conditions are presented. Preactivation of proteins with divinylsulfone (DVS) permits peptide conjugation through either amino, hydroxyl or sulphydryl groups depending on the coupling pH used
Julian P Sefrin et al.
Frontiers in immunology, 10, 1962-1962 (2019-09-27)
Anti-tumor immunity is limited by a number of factors including the lack of fully activated T-cells, insufficient antigenic stimulation and the immune-suppressive tumor microenvironment. We addressed these hurdles by developing a novel class of immunoconjugates, Antibody-Targeted Pathogen-derived Peptides (ATPPs), which
E S Rector et al.
Journal of immunological methods, 24(3-4), 321-336 (1978-01-01)
A novel procedure for the synthesis of well-defined protein-protein conjugates is described using ovalbumin (OA) and IgG as test proteins. This procedure involves the highly selective and rapid reaction of alkyl halide and sulfhydryl groups, which have been grafted, respectively
Elena V Piletska et al.
Journal of molecular recognition : JMR, 33(4), e2824-e2824 (2019-11-20)
A library of 17 nanoparticles made of acrylate and methacrylate copolymers is prepared, characterized, and screened against six epitopes of adeno-associated viruses (AAV)-neutralizing antibodies to assess their affinity and specificity. Peptide epitopes are immobilized onto the surface of glass beads
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service