D188700
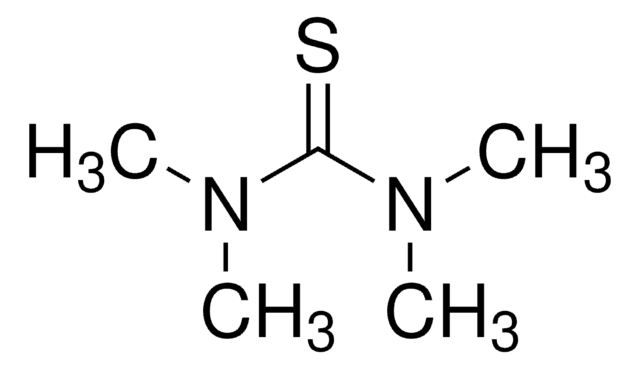
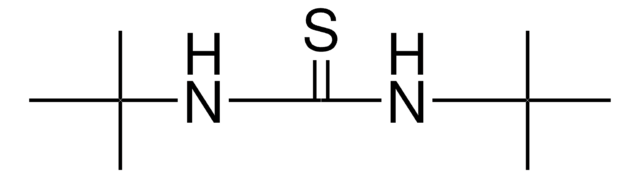
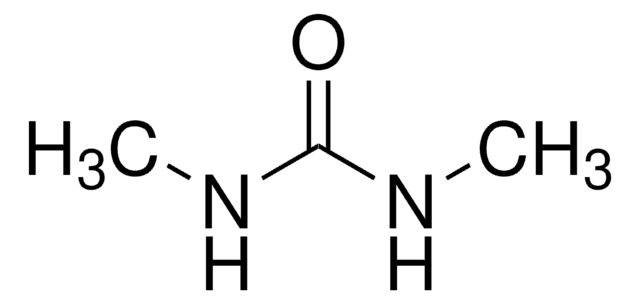
N,N′-Dimethylthiourea
99%
Synonym(s):
1,3-Dimethyl-2-thiourea, 1,3-Dimethylisothiourea, Dimethylthiocarbamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3NHCSNHCH3
CAS Number:
Molecular Weight:
104.17
Beilstein:
605454
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
crystals
SMILES string
CNC(=S)NC
InChI
1S/C3H8N2S/c1-4-3(6)5-2/h1-2H3,(H2,4,5,6)
InChI key
VLCDUOXHFNUCKK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N,N′-Dimethylthiourea, also known as 1,3-Dimethylisothiourea, is commonly used in organic reactions to synthesize N-containing compounds such as N-acyl-substituted 1,1-diaminoethylenes, amidines, ureas, and thioureas. It also acts as a nucleophilic catalyst in the bromination of alcohols using N-bromosuccinimide (NBS), during the conversion to the corresponding bromides.
Application
- Pharmaceutical Analysis: Mao et al. developed an itaconic acid-based organic-polymer monolithic column for hydrophilic capillary electrochromatography, which was used in pharmaceutical analysis. N,N′-Dimethylthiourea could be utilized to improve the column′s performance by acting as a potential organic modifier to enhance the separation processes (Mao et al., 2024).
- Agricultural Research: Li et al. examined the effect of the reactive oxygen scavenger N,N′-Dimethylthiourea on seed germination and radicle elongation of maize. This research could help in understanding how this chemical can be used to influence plant growth under stress conditions, potentially improving agricultural productivity (Li et al., 2023).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Emily E Chea et al.
Biophysical journal, 118(1), 128-137 (2019-12-08)
Cytochrome c (cyt c) is known for its role in the electron transport chain but transitions to a peroxidase-active state upon exposure to oxidative species. The peroxidase activity ultimately results in the release of cyt c into the cytosol for
Rostyslav R Panchuk et al.
Free radical biology & medicine, 106, 134-147 (2017-02-13)
Landomycin E (LE) is an angucycline antibiotic produced by Streptomyces globisporus. Previously, we have shown a broad anticancer activity of LE which is, in contrast to the structurally related and clinically used anthracycline doxorubicin (Dx), only mildly affected by multidrug
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service